Abstract
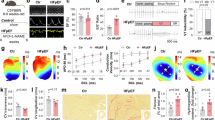
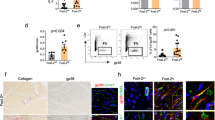
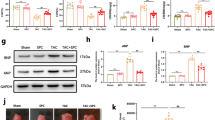
Cardiac fibroblasts (CFs) activation plays a crucial role in cardiac remodeling. However, the molecular mechanisms underlying the fibroblast-to-myofibroblast transition remain largely unknown. Here we found elevated Foxm1 expression in human heart failure (HF) samples as well as in the mouse cardiac remodeling model. CFs were the primary cell type responsible for Foxm1 upregulation. Foxm1 genetic knockout in CFs or myofibroblasts significantly attenuated TAC-induced cardiac remodeling and HF. Conversely, conditional overexpression of Foxm1 in CFs resulted in more severe pathological cardiac remodeling and dysfunction by TAC. Combined RNA-sequencing and MS analysis revealed that Foxm1 promoted p38 mitogen-activated protein kinase (MAPK) signalling pathway. Mechanically, Foxm1 not only upregulates USP10 to reduce the ubiquitination and degradation of p38γ but also directly drives the expression of MKK6 to increase the phosphorylation levels of p38, thereby acting as a dual amplifier for p38 activation. Genetic knockout of p38γ ameliorated the exacerbated TAC-induced cardiac remodeling in mice with Foxm1 overexpression in CFs. Our findings suggest that targeting the Foxm1-USP10/MKK6-p38γ MAPK axis may represent a new potential therapeutic strategy against pathological cardiac remodeling and HF.

Foxm1 promotes the CFs activation during TAC-induced pathological cardiac remodeling through modulation of the USP10/MKK6-p38γ MAPK signalling pathway. Pro-fibrotic stimuli increase Foxm1 expression, which upregulates USP10 to reduce the ubiquitination and degradation of p38γ but also directly drives the expression of MKK6 to increase the phosphorylation levels of p38, thereby acting as a dual amplifier for p38 activation and promoting fibroblast-to-myofibroblast transition. Moreover, genetic knockout of p38γ mitigates exacerbated TAC-induced pathological cardiac remodeling in mice with Foxm1 overexpression in CFs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The sequencing data and proteomics data have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE248447 and ProteomeXchange under accession number PXD050300. Full-length, uncropped original Western blots have been uploaded. All other data presented in the paper are available from the corresponding author upon reasonable request.
References
Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018;15:83–96.
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–78.
Perestrelo AR, Silva AC, Oliver-De La Cruz J, Martino F, Horvath V, Caluori G, et al. Multiscale analysis of extracellular matrix remodeling in the failing heart. Circ Res. 2021;128:24–38.
Mewhort HEM, Svystonyuk DA, Turnbull JD, Teng G, Belke DD, Guzzardi DG, et al. Bioactive extracellular matrix scaffold promotes adaptive cardiac remodeling and repair. JACC Basic Transl Sci. 2017;2:450–64.
Fraccarollo D, Galuppo P, Motschenbacher S, Ruetten H, Schafer A, Bauersachs J. Soluble guanylyl cyclase activation improves progressive cardiac remodeling and failure after myocardial infarction. Cardioprotection over ACE inhibition. Basic Res Cardiol. 2014;109:421.
Remes A, Wagner AH, Schmiedel N, Heckmann M, Ruf T, Ding L, et al. AAV-mediated expression of NFAT decoy oligonucleotides protects from cardiac hypertrophy and heart failure. Basic Res Cardiol. 2021;116:38.
Luo Y, Jiang N, May HI, Luo X, Ferdous A, Schiattarella GG, et al. Cooperative binding of ETS2 and NFAT links Erk1/2 and calcineurin signaling in the pathogenesis of cardiac hypertrophy. Circulation. 2021;144:34–51.
Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830–50.
Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, et al. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114:1269–76.
Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–74.
Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36.
Ustiyan V, Wang IC, Ren X, Zhang Y, Snyder J, Xu Y, et al. Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus. Dev Biol. 2009;336:266–79.
Ramakrishna S, Kim IM, Petrovic V, Malin D, Wang IC, Kalin TV, et al. Myocardium defects and ventricular hypoplasia in mice homozygous null for the Forkhead Box M1 transcription factor. Dev Dyn. 2007;236:1000–13.
Bolte C, Zhang Y, Wang IC, Kalin TV, Molkentin JD, Kalinichenko VV. Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PLoS One. 2011;6:e22217.
Yang J, Feng X, Zhou Q, Cheng W, Shang C, Han P, et al. Pathological Ace2-to-Ace enzyme switch in the stressed heart is transcriptionally controlled by the endothelial Brg1-FoxM1 complex. Proc Natl Acad Sci USA. 2016;113:E5628–5635.
Huang X, Zhang X, Machireddy N, Evans CE, Trewartha SD, Hu G, et al. Endothelial FoxM1 reactivates aging-impaired endothelial regeneration for vascular repair and resolution of inflammatory lung injury. Sci Transl Med. 2023;15:eabm5755.
Gage MC, Becares N, Louie R, Waddington KE, Zhang Y, Tittanegro TH, et al. Disrupting LXRalpha phosphorylation promotes FoxM1 expression and modulates atherosclerosis by inducing macrophage proliferation. Proc Natl Acad Sci USA. 2018;115:E6556–E6565.
Sengupta A, Kalinichenko VV, Yutzey KE. FoxO1 and FoxM1 transcription factors have antagonistic functions in neonatal cardiomyocyte cell-cycle withdrawal and IGF1 gene regulation. Circ Res. 2013;112:267–77.
Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–43.
Raghavan A, Zhou G, Zhou Q, Ibe JC, Ramchandran R, Yang Q, et al. Hypoxia-induced pulmonary arterial smooth muscle cell proliferation is controlled by forkhead box M1. Am J Respir Cell Mol Biol. 2012;46:431–6.
Pal-Ghosh R, Xue D, Warburton R, Hill N, Polgar P, Wilson JL. CDC2 is an important driver of vascular smooth muscle cell proliferation via FOXM1 and PLK1 in pulmonary arterial hypertension. Int J Mol Sci. 2021;22.
Huang X, Zhang X, Zhao DX, Yin J, Hu G, Evans CE, et al. Endothelial hypoxia-inducible factor-1alpha is required for vascular repair and resolution of inflammatory lung injury through forkhead box protein M1. Am J Pathol. 2019;189:1664–79.
Dai Z, Zhu MM, Peng Y, Jin H, Machireddy N, Qian Z, et al. Endothelial and smooth muscle cell interaction via FoxM1 signaling mediates vascular remodeling and pulmonary hypertension. Am J Respir Crit Care Med. 2018;198:788–802.
Dai J, Zhou Q, Tang H, Chen T, Li J, Raychaudhuri P, et al. Smooth muscle cell-specific FoxM1 controls hypoxia-induced pulmonary hypertension. Cell Signal. 2018;51:119–29.
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260.
Li Y, Li Z, Zhang C, Li P, Wu Y, Wang C, et al. Cardiac fibroblast-specific activating transcription factor 3 protects against heart failure by suppressing MAP2K3-p38 signaling. Circulation. 2017;135:2041–57.
Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta). J Biol Chem. 1996;271:17920–6.
Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11.
Wang J, Seth A, McCulloch CA. Force regulates smooth muscle actin in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2000;279:H2776–2785.
Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, et al. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation. 2017;136:549–61.
Zhao D, Zhong G, Li J, Pan J, Zhao Y, Song H, et al. Targeting E3 ubiquitin ligase WWP1 prevents cardiac hypertrophy through destabilizing DVL2 via inhibition of K27-linked ubiquitination. Circulation. 2021;144:694–711.
Ying X, Zhao Y, Yao T, Yuan A, Xu L, Gao L, et al. Novel protective role for ubiquitin-specific protease 18 in pathological cardiac remodeling. Hypertension. 2016;68:1160–70.
Bolte C, Zhang Y, York A, Kalin TV, Schultz JelJ, Molkentin JD, et al. Postnatal ablation of Foxm1 from cardiomyocytes causes late onset cardiac hypertrophy and fibrosis without exacerbating pressure overload-induced cardiac remodeling. PLoS One. 2012;7:e48713.
Bourgeois A, Lambert C, Habbout K, Ranchoux B, Paquet-Marceau S, Trinh I, et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. J Mol Med. 2018;96:223–35.
Snider JC, Riley LA, Mallory NT, Bersi MR, Umbarkar P, Gautam R, et al. Targeting 5-HT2B receptor signaling prevents border zone expansion and improves microstructural remodeling after myocardial infarction. Circulation. 2021;143:1317–30.
Huo JL, Jiao L, An Q, Chen X, Qi Y, Wei B, et al. Myofibroblast deficiency of LSD1 alleviates TAC-induced heart failure. Circ Res. 2021;129:400–13.
Penke LR, Speth JM, Dommeti VL, White ES, Bergin IL, Peters-Golden M. FOXM1 is a critical driver of lung fibroblast activation and fibrogenesis. J Clin Investig. 2018;128:2389–405.
Im J, Lawrence J, Seelig D, Nho RS. FoxM1-dependent RAD51 and BRCA2 signaling protects idiopathic pulmonary fibrosis fibroblasts from radiation-induced cell death. Cell Death Dis. 2018;9:584.
Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–47.
Tian S, Lei I, Gao W, Liu L, Guo Y, Creech J, et al. HDAC inhibitor valproic acid protects heart function through Foxm1 pathway after acute myocardial infarction. EBioMedicine. 2019;39:83–94.
Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91–112.
Schlittler M, Pramstaller PP, Rossini A, De Bortoli M. Myocardial fibrosis in hypertrophic cardiomyopathy: a perspective from fibroblasts. Int J Mol Sci. 2023;24.
Bang C, Antoniades C, Antonopoulos AS, Eriksson U, Franssen C, Hamdani N, et al. Intercellular communication lessons in heart failure. Eur J Heart Fail. 2015;17:1091–103.
Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–63.
Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, et al. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol. 2004;276:74–88.
Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem. 2005;280:22278–86.
Huang X, Zhao YY. Transgenic expression of FoxM1 promotes endothelial repair following lung injury induced by polymicrobial sepsis in mice. PLoS One. 2012;7:e50094.
Zhao YD, Huang X, Yi F, Dai Z, Qian Z, Tiruppathi C, et al. Endothelial FoxM1 mediates bone marrow progenitor cell-induced vascular repair and resolution of inflammation following inflammatory lung injury. Stem Cells. 2014;32:1855–64.
Bolte C, Ustiyan V, Ren X, Dunn AW, Pradhan A, Wang G, et al. Nanoparticle Delivery of Proangiogenic Transcription Factors into the Neonatal Circulation Inhibits Alveolar Simplification Caused by Hyperoxia. Am J Respir Crit Care Med. 2020;202:100–11.
Liu J, Zhuang T, Pi J, Chen X, Zhang Q, Li Y, et al. Endothelial forkhead box transcription factor P1 regulates pathological cardiac remodeling through transforming growth factor-beta1-endothelin-1 signal pathway. Circulation. 2019;140:665–80.
Xie Y, Li Y, Kong Y. OPN induces FoxM1 expression and localization through ERK 1/2, AKT, and p38 signaling pathway in HEC-1A cells. Int J Mol Sci. 2014;15:23345–58.
Goda C, Balli D, Black M, Milewski D, Le T, Ustiyan V, et al. Loss of FOXM1 in macrophages promotes pulmonary fibrosis by activating p38 MAPK signaling pathway. PLoS Genet. 2020;16:e1008692.
Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, et al. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117:545–52.
See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–89.
Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, et al. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–502.
Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, et al. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. J Card Fail. 2005;11:74–81.
Bhattacharya U, Neizer-Ashun F, Mukherjee P, Bhattacharya R. When the chains do not break: the role of USP10 in physiology and pathology. Cell Death Dis. 2020;11:1033.
Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–97.
An S, Zhao LP, Shen LJ, Wang S, Zhang K, Qi Y, et al. USP18 protects against hepatic steatosis and insulin resistance through its deubiquitinating activity. Hepatology. 2017;66:1866–84.
Acknowledgements
This work was supported by the China National Natural Science Foundation (grant number 82470271 to Shuai Song), Basic Science Center Project (grant number T2288101 to Junbo Ge), the National Natural Science Foundation (grant number 82130010 to Aijun Sun), the Shanghai Clinical Research Center for Interventional Medicine (grant number 19MC1910300 to Junbo Ge), the National Natural Science Foundation (grant number 82370261 to Hao Lu) and the National Natural Science Foundation (grant number 82470377 to Xiaochun Zhang). This work was supported by the Medical Science Data Center of Fudan University.
Author information
Authors and Affiliations
Contributions
Aijun Sun and Junbo Ge conceived and designed the study. Shuai Song, Xiaokai Zhang, Zihang Huang, Linqi Zeng, Fengze Cai, Tongyao Wang, Jiahao Guo, Mohan Li, Chenyan Liu, Yining Song, Hao Lu, Xinyu Weng, Li Shen, Xiaochun Zhang and Ping Zhu performed the experiments and analysed the data. Shuai Song and Xiaokai Zhang drafted the manuscript. Shuyang Lu provided the clinical samples. Aijun Sun and Junbo Ge provided critical revisions of important intellectual content within the manuscript and provided final approval of the version of the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, S., Zhang, X., Huang, Z. et al. Cardiac fibroblast Foxm1 deficiency prevents pressure overload-induced cardiac remodeling via the Usp10/MKK6-p38γ MAPK axis. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01609-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41418-025-01609-1