Abstract
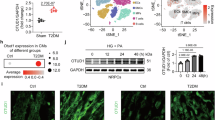
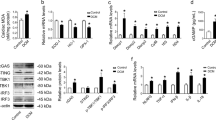
Diabetic cardiomyopathy (DCM) is a leading cause of diabetes-related mortality. Identifying new functional proteins in DCM pathology and elucidating the underlying mechanisms may provide new therapeutic targets for this disease. Here, we observed that the expression of the deubiquitinating enzyme USP13 was significantly downregulated in DCM mouse heart tissues. We discovered that the expression of USP13 was predominantly localized in cardiomyocytes. Cardiomyocyte-specific knockout of USP13 exacerbated myocardial injury in both type I and type II diabetic mice. Conversely, overexpression of USP13 in cardiomyocytes via recombinant adeno-associated virus 9 (AAV9) showed therapeutic effects against DCM in mice. Interestingly, using co-precipitation and LC-MS/MS analysis, we identified the NOD-like receptor family pyrin domain containing 3 (NLRP3) as a target protein of USP13 in cardiomyocytes. Mechanistically, we have illustrated that USP13 removes the K63-linked ubiquitin chain at K557 of NLRP3 to inhibit NLRP3-ASC interaction, thereby inhibiting ASC polymerization and the activation of NLRP3 inflammasome complex, ultimately alleviating pyroptosis in HG + PA challenged cardiomyocytes. Importantly, we showed that the cardioprotective effects of USP13 overexpression depended on NLRP3, as evidenced by the loss of protection in NLRP3-deficient diabetic mice. Taken together, this study identifies the protective impact and molecular regulation of USP13 in DCM pathology, uncovering a novel cardiomyocyte-specific USP13-NLRP3 axis in DCM.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in this study are presented in this manuscript or the supplementary information. The materials described in this study are either commercially available or available upon reasonable request from the corresponding authors.
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diab Res Clin Pract. 2019;157:107843.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–38.
Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019;124:1160–2.
Yuan Q, Sun Y, Yang F, Yan D, Shen M, Jin Z, et al. CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes. Signal Transduct Target Ther. 2023;8:99.
Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71:339–51.
Li X, Du N, Zhang Q, Li J, Chen X, Liu X, et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479.
Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21:193–201.
Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18:579–86.
Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–29.
Yalcinkaya M, Liu W, Thomas LA, Olszewska M, Xiao T, Abramowicz S, et al. BRCC3-mediated NLRP3 deubiquitylation promotes inflammasome activation and atherosclerosis in Tet2 clonal hematopoiesis. Circulation. 2023;148:1764–77.
Dhingra R, Rabinovich-Nikitin I, Rothman S, Guberman M, Gang H, Margulets V, et al. Proteasomal degradation of TRAF2 mediates mitochondrial dysfunction in doxorubicin-cardiomyopathy. Circulation. 2022;146:934–54.
Chu LK, Cao X, Wan L, Diao Q, Zhu Y, Kan Y, et al. Autophagy of OTUD5 destabilizes GPX4 to confer ferroptosis-dependent kidney injury. Nat Commun. 2023;14:8393.
Liu LB, Huang SH, Qiu HL, Cen XF, Guo YY, Li D, et al. Limonin stabilises sirtuin 6 (SIRT6) by activating ubiquitin specific peptidase 10 (USP10) in cardiac hypertrophy. Br J Pharm. 2022;179:4516–33.
Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao Y, et al. USP25 ameliorates pathological cardiac hypertrophy by stabilizing SERCA2a in cardiomyocytes. Circ Res. 2023;132:465–80.
Han J, Lin L, Fang Z, Ye B, Han X, Xu J, et al. Cardiomyocyte-derived USP28 negatively regulates antioxidant response and promotes cardiac hypertrophy via deubiquitinating TRIM21. Theranostics. 2024;14:6236–48.
Wang M, Han X, Yu T, Wang M, Luo W, Zou C, et al. OTUD1 promotes pathological cardiac remodeling and heart failure by targeting STAT3 in cardiomyocytes. Theranostics. 2023;13:2263–80.
Han J, Fang Z, Han B, Ye B, Lin W, Jiang Y, et al. Deubiquitinase JOSD2 improves calcium handling and attenuates cardiac hypertrophy and dysfunction by stabilizing SERCA2a in cardiomyocytes. Nat Cardiovasc Res. 2023;2:764–77.
Sun H, Zhang Q, Jing YY, Zhang M, Wang HY, Cai Z, et al. USP13 negatively regulates antiviral responses by deubiquitinating STING. Nat Commun. 2017;8:15534.
Xie W, Jin S, Cui J. The NEDD4-USP13 axis facilitates autophagy via deubiquitinating PIK3C3. Autophagy. 2020;16:1150–1.
Wang Q, Sun Z, Xia W, Sun L, Du Y, Zhang Y, et al. Role of USP13 in physiology and diseases. Front Mol Biosci. 2022;9:977122.
Han C, Yang L, Choi HH, Baddour J, Achreja A, Liu Y, et al. Amplification of USP13 drives ovarian cancer metabolism. Nat Commun. 2016;7:13525.
Madiraju C, Novack JP, Reed JC, Matsuzawa SI. K63 ubiquitination in immune signaling. Trends Immunol. 2022;43:148–62.
Zhao X, Fiske B, Kawakami A, Li J, Fisher DE. Regulation of MITF stability by the USP13 deubiquitinase. Nat Commun. 2011;2:414.
Xie SY, Liu SQ, Zhang T, Shi WK, Xing Y, Fang WX, et al. USP28 serves as a key suppressor of mitochondrial morphofunctional defects and cardiac dysfunction in the diabetic heart. Circulation. 2024;149:684–706.
Yan M, Su L, Wu K, Mei Y, Liu Z, Chen Y, et al. USP7 promotes cardiometabolic disorders and mitochondrial homeostasis dysfunction in diabetic mice via stabilizing PGC1b. eta Pharm Res. 2024;205:107235.
Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34:101523.
Liu C, Yao Q, Hu T, Cai Z, Xie Q, Zhao J, et al. Cathepsin B deteriorates diabetic cardiomyopathy induced by streptozotocin via promoting NLRP3-mediated pyroptosis. Mol Ther Nucleic Acids. 2022;30:198–207.
Wang J, Li Y, Li L, Liang H, Ye H, Kang P, et al. Effect of NLRP3 gene knockdown on pyroptosis and ferroptosis in diabetic cardiomyopathy injury. BMC Cardiovasc Disord. 2024;24:351.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Chan AHP, Moore MJ, Grant AJ, Lam YTM, Darnell MV, Michael PL, et al. Selective immunosuppression targeting the NLRP3 inflammasome mitigates the foreign body response to implanted biomaterials while preserving angiogenesis. Adv Health Mater. 2023;12:e2301571.
Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–8.
Zhao W, Cao Y, Chen Y, Chen Y, Wang T, Li L, et al. NLRP3 Regulates Mandibular Healing through Interaction with UCHL5 in MSCs. Int J Biol Sci. 2023;19:936–49.
Bednash JS, Johns F, Patel N, Smail TR, Londino JD, Mallampalli RK. The deubiquitinase STAMBP modulates cytokine secretion through the NLRP3 inflammasome. Cell Signal. 2021;79:109859.
Wang W, Hu D, Feng Y, Wu C, Song Y, Liu W, et al. Paxillin mediates ATP-induced activation of P2X7 receptor and NLRP3 inflammasome. BMC Biol. 2020;18:182.
Henning NJ, Boike L, Spradlin JN, Ward CC, Liu G, Zhang E, et al. Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat Chem Biol. 2022;18:412–21.
Park YJ, Dodantenna N, Kim Y, Kim TH, Lee HS, Yoo YS, et al. MARCH5-dependent NLRP3 ubiquitination is required for mitochondrial NLRP3-NEK7 complex formation and NLRP3 inflammasome activation. EMBO J. 2023;42:e113481.
Tang J, Tu S, Lin G, Guo H, Yan C, Liu Q, et al. Sequential ubiquitination of NLRP3 by RNF125 and Cbl-b limits inflammasome activation and endotoxemia. J Exp Med. 2020;217:e20182091.
Ni J, Guan C, Liu H, Huang X, Yue J, Xiang H, et al. Ubc13 promotes K63-linked polyubiquitination of NLRP3 to activate inflammasome. J Immunol. 2021;206:2376–85.
Liu X, Fang Y, Lv X, Hu C, Chen G, Zhang L, et al. Deubiquitinase OTUD6A in macrophages promotes intestinal inflammation and colitis via deubiquitination of NLRP3. Cell Death Differ. 2023;30:1457–71.
Liu T, Wang L, Liang P, Wang X, Liu Y, Cai J, et al. USP19 suppresses inflammation and promotes M2-like macrophage polarization by manipulating NLRP3 function via autophagy. Cell Mol Immunol. 2021;18:2431–42.
Emmerich CH, Cohen P. Optimising methods for the preservation, capture and identification of ubiquitin chains and ubiquitylated proteins by immunoblotting. Biochem Biophys Res Commun. 2015;466:1–14.
Acknowledgements
We are grateful to Mengxin Zhang, Lingli Hou, Tongliang Huang, Yanni Dong, and Jiansong Lin from the scientific research center of Wenzhou Medical University for their help in echocardiography and immunofluorescence experiment.
Funding
This study was supported by the National Natural Science Foundation of China (U24A20814 to GL, 82361138563 to YW, and 82270282 to PS) and the Joint Funds of the Zhejiang Provincial Natural Science Foundation of China under Grant No. LHDMY23H310001 to PS.
Author information
Authors and Affiliations
Contributions
GL and SP contributed to the literature search, study design, and manuscript revision. DX, JH, BY, LL, YJ, JC, and YZ, performed the experiments and analyzed the data. YW provided technical help. DX, JH, and BY participated in the drafting of the article. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, D., Han, J., Ye, B. et al. USP13 ameliorates diabetic cardiomyopathy via deubiquitinating NLRP3 and inhibiting pyroptosis in cardiomyocytes. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01612-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41418-025-01612-6