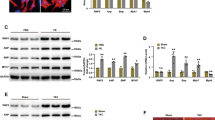
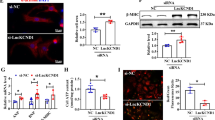
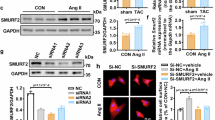
Abstract
Pathological cardiac hypertrophy serves as an independent risk factor for heart failure, which is the final stage of numerous cardiovascular diseases. However, the molecular regulatory mechanisms underlying this pathological process are still poorly characterized. The ubiquitin-proteasome system (UPS) is known to influence the development of pathological cardiac hypertrophy by precisely controlling protein function, localization, and proteostasis. The E3 ubiquitin ligase ring finger protein 220 (RNF220), a component of the UPS, was chosen as the research subject to investigate its role in pathological cardiac hypertrophy. Using Ang II-induced cardiac hypertrophy models combined with RNF220 knockout mice, RNF220 overexpression mice, and primary cardiomyocytes to examine the molecular mechanisms by which RNF220 governs pathological cardiac hypertrophy. We found that RNF220 deficiency promotes resistance to angiotensin II infusion by suppressing myocardial hypertrophy and fibrosis, whereas RNF220 overexpression aggravated cardiac dysfunction and hypertrophic responses. Moreover, using proteomic mass spectrometry and co-immunoprecipitation (Co-IP) experiments, we identified a functional interaction between RNF220 and STAT3. Mechanistically, RNF220 directly binds to the SH2 and TAD structural domains of STAT3 via its N-terminal domain, specifically facilitating K63-linked polyubiquitination at lysine residues 615, 626, 631, and 642 of STAT3, thereby stabilizing its protein to drive pro-hypertrophic responses. Critical rescue experiments demonstrated that STAT3 inhibitors or gene silencing effectively restored the ventricular hypertrophy phenotype caused by RNF220 overexpression. Collectively, these findings reveal a novel mechanism by which RNF220 drives pathological myocardial hypertrophy by regulating STAT3 ubiquitination, indicating a potential therapeutic target for heart failure intervention.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–71.
Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407.
Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens. 2008;21:500–8.
Zhang N, Zhang Y, Chen Y, Qian H, Wu B, Lu S, et al. BAF155 promotes cardiac hypertrophy and fibrosis through inhibition of WWP2-mediated PARP1 ubiquitination. Cell Discov. 2023;9:46.
Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao Y, et al. USP25 Ameliorates Pathological Cardiac Hypertrophy by Stabilizing SERCA2a in Cardiomyocytes. Circ Res. 2023;132:465–80.
Han J, Fang Z, Han B, Ye B, Lin W, Jiang Y, et al. Deubiquitinase JOSD2 improves calcium handling and attenuates cardiac hypertrophy and dysfunction by stabilizing SERCA2a in cardiomyocytes. Nat Cardiovasc Res. 2023;2:764–77.
Sosic I, Bricelj A, Steinebach C. E3 ligase ligand chemistries: from building blocks to protein degraders. Chem Soc Rev. 2022;51:3487–534.
Tang X, Liu X, Sha X, Zhang Y, Zu Y, Fan Q, et al. NEDD4-Mediated GSNOR Degradation Aggravates Cardiac Hypertrophy and Dysfunction. Circ Res. 2025;136:422–38.
van de Vegte YJ, Tegegne BS, Verweij N, Snieder H, van der Harst P. Genetics and the heart rate response to exercise. Cell Mol Life Sci. 2019;76:2391–409.
Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy–relation to left ventricular mass and etiology. Am Heart J. 2006;151:829–36.
Shanks J, Abukar Y, Lever NA, Pachen M, LeGrice IJ, Crossman DJ, et al. Reverse re-modelling chronic heart failure by reinstating heart rate variability. Basic Res Cardiol. 2022;117:4.
Ozel E, Tastan A, Ozturk A, Ozcan EE. Relationship between Sympathetic Overactivity and Left Ventricular Hypertrophy in Resistant Hypertension. Hellenic J Cardiol. 2015;56:501–6.
Tegegne BS, Said MA, Ani A, van Roon AM, Shah S, de Geus EJC, et al. Phenotypic but not genetically predicted heart rate variability associated with all-cause mortality. Commun Biol. 2023;6:1013.
Ma P, Yang X, Kong Q, Li C, Yang S, Li Y, et al. The ubiquitin ligase RNF220 enhances canonical Wnt signaling through USP7-mediated deubiquitination of beta-catenin. Mol Cell Biol. 2014;34:4355–66.
Deng T, Zhong P, Lou R, Yang X. RNF220 promotes gastric cancer growth and stemness via modulating the USP22/wnt/beta-catenin pathway. Tissue Cell. 2023;83:102123.
Azhdari M, Zur Hausen A. Wnt/beta-catenin and notch signaling pathways in cardiovascular disease: Mechanisms and therapeutics approaches. Pharmacol Res. 2025;211:107565.
Nayakanti SR, Friedrich A, Sarode P, Jafari L, Maroli G, Boehm M, et al. Targeting Wnt-ss-Catenin-FOSL Signaling Ameliorates Right Ventricular Remodeling. Circ Res. 2023;132:1468–85.
Zhao Y, Wang C, Hong X, Miao J, Liao Y, Hou FF, et al. Wnt/beta-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. 2019;95:815–29.
Nakao S, Tsukamoto T, Ueyama T, Kawamura T. STAT3 for Cardiac Regenerative Medicine: Involvement in Stem Cell Biology, Pathophysiology, and Bioengineering. Int J Mol Sci. 2020;21:1937.
Pipicz M, Demjan V, Sarkozy M, Csont T. Effects of Cardiovascular Risk Factors on Cardiac STAT3. Int J Mol Sci. 2018;19:3572.
Haghikia A, Stapel B, Hoch M, Hilfiker-Kleiner D. STAT3 and cardiac remodeling. Heart Fail Rev. 2011;16:35–47.
Kleinbongard P. Perspective: mitochondrial STAT3 in cardioprotection. Basic Res Cardiol. 2023;118:32.
Comita S, Femmino S, Thairi C, Alloatti G, Boengler K, Pagliaro P, et al. Regulation of STAT3 and its role in cardioprotection by conditioning: focus on non-genomic roles targeting mitochondrial function. Basic Res Cardiol. 2021;116:56.
Avalle L, Poli V. Nucleus, Mitochondrion, or Reticulum? STAT3 a La Carte. Int J Mol Sci. 2018;19:2820.
Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–95.
Liu Z, Bian X, Li L, Liu L, Feng C, Wang Y, et al. SENP1-Mediated HSP90ab1 DeSUMOylation in Cardiomyocytes Prevents Myocardial Fibrosis by Paracrine Signaling. Adv Sci. 2024;11:e2400741.
Hilfiker-Kleiner D, Limbourg A, Drexler H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc Med. 2005;15:152–7.
Avalle L, Camporeale A, Morciano G, Caroccia N, Ghetti E, Orecchia V, et al. STAT3 localizes to the ER, acting as a gatekeeper for ER-mitochondrion Ca(2+) fluxes and apoptotic responses. Cell Death Differ. 2019;26:932–42.
Wang P, Xu S, Xu J, Xin Y, Lu Y, Zhang H, et al. Elevated MCU Expression by CaMKIIdeltaB Limits Pathological Cardiac Remodeling. Circulation. 2022;145:1067–83.
Ma P, Song NN, Li Y, Zhang Q, Zhang L, Zhang L, et al. Fine-Tuning of Shh/Gli Signaling Gradient by Non-proteolytic Ubiquitination during Neural Patterning. Cell Rep. 2019;28:541–53.e4.
Lipkowitz S, Weissman AM. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer. 2011;11:629–43.
Cai C, Tang YD, Zhai J, Zheng C. The RING finger protein family in health and disease. Signal Transduct Target Ther. 2022;7:300.
Yuan L, Bu S, Du M, Wang Y, Ju C, Huang D, et al. RNF207 exacerbates pathological cardiac hypertrophy via post-translational modification of TAB1. Cardiovasc Res. 2023;119:183–94.
Yang LL, Xiao WC, Li H, Hao ZY, Liu GZ, Zhang DH, et al. E3 ubiquitin ligase RNF5 attenuates pathological cardiac hypertrophy through STING. Cell Death Dis. 2022;13:889.
Guo X, Ma P, Li Y, Yang Y, Wang C, Xu T, et al. RNF220 mediates K63-linked polyubiquitination of STAT1 and promotes host defense. Cell Death Differ. 2021;28:640–56.
Haghikia A, Ricke-Hoch M, Stapel B, Gorst I, Hilfiker-Kleiner D. STAT3, a key regulator of cell-to-cell communication in the heart. Cardiovasc Res. 2014;102:281–9.
Ye S, Luo W, Khan ZA, Wu G, Xuan L, Shan P, et al. Celastrol Attenuates Angiotensin II-Induced Cardiac Remodeling by Targeting STAT3. Circ Res. 2020;126:1007–23.
Wang M, Han X, Yu T, Wang M, Luo W, Zou C, et al. OTUD1 promotes pathological cardiac remodeling and heart failure by targeting STAT3 in cardiomyocytes. Theranostics. 2023;13:2263–80.
Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62.
Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer. 2020;19:145.
Martinez-Ferriz A, Ferrando A, Fathinajafabadi A, Farras R. Ubiquitin-mediated mechanisms of translational control. Semin Cell Dev Biol. 2022;132:146–54.
Cho JJ, Xu Z, Parthasarathy U, Drashansky TT, Helm EY, Zuniga AN, et al. Hectd3 promotes pathogenic Th17 lineage through Stat3 activation and Malt1 signaling in neuroinflammation. Nat Commun. 2019;10:701.
Ruan HH, Zhang Z, Wang SY, Nickels LM, Tian L, Qiao JJ, et al. Tumor Necrosis Factor Receptor-Associated Factor 6 (TRAF6) Mediates Ubiquitination-Dependent STAT3 Activation upon Salmonella enterica Serovar Typhimurium Infection. Infect Immun. 2017;85:e00081-17.
Ao YQ, Gao J, Jin C, Wang S, Zhang LC, Deng J, et al. ASCC3 promotes the immunosuppression and progression of non-small cell lung cancer by impairing the type I interferon response via CAND1-mediated ubiquitination inhibition of STAT3. J Immunother Cancer. 2023;11:e007766.
Kasembeli MM, Kaparos E, Bharadwaj U, Allaw A, Khouri A, Acot B, et al. Aberrant function of pathogenic STAT3 mutant proteins is linked to altered stability of monomers and homodimers. Blood. 2023;141:1411–24.
Bocchini CE, Nahmod K, Katsonis P, Kim S, Kasembeli MM, Freeman A, et al. Protein stabilization improves STAT3 function in autosomal dominant hyper-IgE syndrome. Blood. 2016;128:3061–72.
Acknowledgements
We thank Mengmeng Zhao (Central Hospital Affiliated Shandong First Medical University, Jinan) for ubiquitin plasmids; Tianning Wang (Central Hospital Affiliated Shandong First Medical University, Jinan) for plasmid construction and technical support.
Funding
This work was supported by grants from the Natural Science Foundation of Shandong Province (No. ZR2024MH214) and Qingdao Outstanding Health Professional Development Fund.
Author information
Authors and Affiliations
Contributions
YG designed the study, conducted the major experiments, and drafted the manuscript. ZZ performed the data analysis. XPC, NL and YL assisted with partial experiments. As the project leaders, JG and HYD co-supervised the research, contributed to manuscript writing, provided experimental guidance, and secured funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All animal experiments were approved by the Animal Welfare and Ethics Committee of Jinan Central Hospital (Approval Document No. JNCHIACUC2024-74, JNCHIACUC2024-75) and were conducted in strict accordance with institutional guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Zhao, Z., Chen, X. et al. RNF220 mediates K63-linked polyubiquitination of STAT3 and aggravates pathological cardiac hypertrophy. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01614-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41418-025-01614-4