Abstract
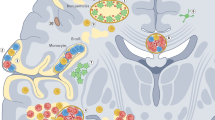
Chemotherapy is essential for cancer management yet frequently accompanied with adverse effects, particularly for temozolomide (TMZ), a frontline chemotherapeutic agent for glioma. Although clinical neurological abnormalities linked to TMZ have been observed, mechanisms underlying TMZ-induced neural impairments remain poorly understood, and effective interventions are lacking. Here, we demonstrated that TMZ chemotherapy induced neurodegenerations that recapitulated pathological features of multiple sclerosis, including demyelination, neuroinflammation and axonal degeneration. In adolescent mice, TMZ treatment resulted in severe white matter damage that spontaneously recovered, whereas in adult mice, moderate myelin damage persisted without recovery within the same timeframe. Importantly, we identified that clobetasol effectively reversed TMZ-induced white matter damage and trends toward anxiety and depression in adult mice by suppressing TMZ-induced AMPK activation and attenuating neuroinflammation, thereby promoting remyelination. Our findings reveal the previously underappreciated neural toxicities associated with TMZ chemotherapy and highlight the therapeutic efficacy of clobetasol in mitigating chemotherapy-induced neural impairment, providing a strategy to enhance the life quality of cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant materials, including the snRNA-seq and bulk RNA-seq data, are available from the corresponding author upon reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024:74:229–63.
DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–53.
Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, et al. Glioma. Nat Rev Dis Prim. 2015;1:1–18.
Berger TR, Wen PY, Lang-Orsini M, Chukwueke UN. World Health Organization 2021 Classification of central nervous system tumors and implications for therapy for adult-type gliomas: a review. JAMA Oncol. 2022;8:1493–501.
Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Mrugala MM, Chamberlain MC. Mechanisms of disease: temozolomide and glioblastoma—look to the future. Nat Clin Pract Oncol. 2008;5:476–86.
Scaringi C, De Sanctis V, Minniti G, Enrici RM. Temozolomide-related hematologic toxicity. Oncol Res Treat. 2013;36:444–9.
Bae SH, Park M-J, Lee MM, Kim TM, Lee S-H, Cho SY, et al. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci. 2014;29:980.
Diamantis N, Banerji U. Antibody-drug conjugates-an emerging class of cancer treatment. Br J Cancer. 2016;114:362–7.
Wang Q, Qi F, Song X, Di J, Zhang L, Zhou Y, et al. A prospective longitudinal evaluation of cognition and depression in postoperative patients with high-grade glioma following radiotherapy and chemotherapy. J Cancer Res Ther. 2018;14:S1048–S51.
Lombardi G, Bergo E, Del Bianco P, Bellu L, Pambuku A, Caccese M, et al. Quality of Life Perception, Cognitive Function, and Psychological Status in a Real-world Population of Glioblastoma Patients Treated With Radiotherapy and Temozolomide: A Single-center Prospective Study. Am J Clin Oncol. 2018;41:1263–71.
Park DY, Tom MC, Chen Y, Tewari S, Ahluwalia MS, Yu JS, et al. Cognitive function after concurrent temozolomide-based chemoradiation therapy in low-grade gliomas. J Neuro-Oncol. 2022;158:341–8.
Abete-Fornara G, Bintintan Socaciu P, Fanizzi C, Fiore G, Locatelli M, Caroli M. Neuropsychological functioning during chemotherapy with temozolomide in high-grade glioma patients: a retrospective single centre study. J Neuro-Oncol. 2023;165:561–8.
Najm FJ, Madhavan M, Zaremba A, Shick E, Karl RT, Factor DC, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–20.
Cen L, Carlson BL, Pokorny JL, Mladek AC, Grogan PT, Schroeder MA, et al. Efficacy of protracted temozolomide dosing is limited in MGMT unmethylated GBM xenograft models. Neuro Oncol. 2013;15:735–46.
Cai W, Maldonado NV, Cui W, Harutyunyan N, Ji L, Sposto R, et al. Activity of irinotecan and temozolomide in the presence of O6-methylguanine-DNA methyltransferase inhibition in neuroblastoma pre-clinical models. Br J Cancer. 2010;103:1369–79.
Elbaz B, Popko B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019;42:263–77.
Jiang W, Yang W, Yang W, Zhang J, Pang D, Gan L, et al. Identification of Tmem10 as a novel late-stage oligodendrocytes marker for detecting hypomyelination. Int J Biol Sci. 2013;10:33.
Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med. 2018;378:169–80.
Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–30.
Yin J, Wang X, Ge X, Ding F, Shi Z, Ge Z, et al. Hypoxanthine phosphoribosyl transferase 1 metabolizes temozolomide to activate AMPK for driving chemoresistance of glioblastomas. Nat Commun. 2023;14:5913.
Liu X, Peng S, Zhao Y, Zhao T, Wang M, Luo L, et al. AMPK Negatively Regulates Peripheral Myelination via Activation of c-Jun. Mol Neurobiol. 2017;54:3554–64.
Guo D, Hildebrandt IJ, Prins RM, Soto H, Mazzotta MM, Dang J, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci USA. 2009;106:12932–7.
Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, et al. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem. 2010;285:40461–71.
Nishiyama A, Shimizu T, Sherafat A, Richardson WD. Life-long oligodendrocyte development and plasticity. Semin Cell Devel Biol. 2021;116:25–37.
Woo MS, Engler JB, Friese MA. The neuropathobiology of multiple sclerosis. Nat Rev Neurosci. 2024;25:493–513.
Simó M, Rifà-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: a systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:1311–21.
Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Stock JVD, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32:480–93.
Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-Oncol. 1995;4:61–6.
van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd, Wall E, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–8.
Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–50.
Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–701.
Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–93.
Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–83.
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. Chemobrain’ in breast carcinoma?: a prologue. Cancer. 2004;101:466–75.
Scherwath A, Mehnert A, Schleimer B, Schirmer L, Fehlauer F, Kreienberg R, et al. Neuropsychological function in high-risk breast cancer survivors after stem-cell supported high-dose therapy versus standard-dose chemotherapy: evaluation of long-term treatment effects. Ann Oncol. 2006;17:415–23.
Mancuso A, Migliorino M, De Santis S, Saponiero A, De Marinis F. Correlation between anemia and functional/cognitive capacity in elderly lung cancer patients treated with chemotherapy. Ann Oncol. 2006;17:146–50.
Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell. 2019;176:43–55 e13.
Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron. 2019;103:250–65 e8.
Kim T, James BT, Kahn MC, Blanco-Duque C, Abdurrob F, Islam MR, et al. Gamma entrainment using audiovisual stimuli alleviates chemobrain pathology and cognitive impairment induced by chemotherapy in mice. Sci Transl Med. 2024;16:eadf4601.
Nguyen LD, Ehrlich BE. Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. EMBO Mol Med. 2020;12:e12075.
Zou J, Zhou L, Du XX, Ji Y, Xu J, Tian J, et al. Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev Cell. 2011;20:97–108.
Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, et al. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J Neurosci. 2014;34:15764–78.
Gonzalez A, Hall MN, Lin SC, Hardie DG. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020;31:472–92.
Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex. 2014;54:33–50.
Vicario N, Spitale FM, Tibullo D, Giallongo C, Amorini AM, Scandura G, et al. Clobetasol promotes neuromuscular plasticity in mice after motoneuronal loss via sonic hedgehog signaling, immunomodulation and metabolic rebalancing. Cell Death Dis. 2021;12:625.
Cully M. Repurposing for remyelination. Nat Rev Drug Discov. 2015;14:383.
Acknowledgements
We thank Dr. Kunlong Zhang for the technical help on confocal imaging, and Dr. Shiping Liao, Zhangyu He and Baichuan Li for the support in mouse experiments.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 82272644 to Y.L., 82372836 to W.Y., and 81571195 to M.C.), and Sichuan Science and Technology Program (No. 2023YFQ0002 to Y.L. and 2023YFSY0042 to W.Y.).
Author information
Authors and Affiliations
Contributions
Conceptualization: Qiuyun Yuan, Wanchun Yang, Yanhui Liu, Mina Chen. Methodology: Qiuyun Yuan, Wanchun Yang, Siliang Chen, Yunbo Yuan, Jingwen Gong, Yue Qin. Investigation: Qiuyun Yuan, Wanchun Yang, Siliang Chen, Jingwen Gong, Tengfei Li, Mingrong Zuo, Yuting Shu, Yuze He, Zhihao Wang, Xiaoqiang Xia, and Yiyuan Cui. Visualization: Qiuyun Yuan, Wanchun Yang, Siliang Chen, Yunbo Yuan. Funding acquisition: Wanchun Yang, Yanhui Liu and Mina Chen. Project administration: Yanhui Liu. Supervision: Yanhui Liu and Mina Chen. Writing – original draft: Qiuyun Yuan and Wanchun Yang. Writing – review & editing: Yanhui Liu and Mina Chen.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All human studies were approved by the Institutional Review Board of West China Hospital of Sichuan University (No. 20241074) and all patients provided written informed consent. For animal works, ethical guidelines for animal experimentation were followed, with approval from the Institutional Animal Care and Use Committees of West China Hospital of Sichuan University (No. 20220228009).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Q., Yang, W., Chen, S. et al. Chemotherapy induces multiple sclerosis-like neuropathologies that can be rescued by clobetasol. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01635-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41418-025-01635-z