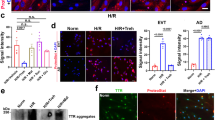
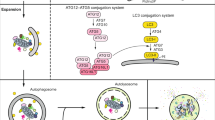
Abstract
Autophagy is a conserved degradation process in eukaryotic cells that is regulated by autophagy-related genes. During autophagy, lysosomes break down cytoplasmic proteins and damaged organelles. This process plays a pivotal role in cell growth and development, protection against metabolic stress and oxidative damage, and the maintenance of cellular homeostasis through the recycling of cellular components. Pregnancy encompasses crucial events such as decidualization, embryo implantation, and fetal growth. Abnormal autophagy has been implicated in several pregnancy complications and can significantly impact both maternal and fetal health. Understanding the relationship between autophagy and complicated pregnancies could open new avenues for potential therapeutic interventions to improve maternal and fetal outcomes. In this review, we summarize the intricate relationship between autophagy and pregnancy complications, elucidate the role of autophagy in gestation, and discuss the clinical significance of autophagy in mitigating or preventing pregnancy-related disorders.
Similar content being viewed by others
References
Deter RL, De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967;33:437–49.
Ornatowski W, Lu Q, Yegambaram M, Garcia AE, Zemskov EA, Maltepe E, et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020;36:101679.
Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, et al. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18:50–72.
Kocak M, Ezazi Erdi S, Jorba G, Maestro I, Farrés J, Kirkin V, et al. Targeting autophagy in disease: established and new strategies. Autophagy. 2022;18:473–95.
Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–34.
Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17:647–61.
Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–37.
Mizushima N, Longo DL, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–76.
Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41.
Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21:439–58.
Fleming A, Bourdenx M, Fujimaki M, Karabiyik C, Krause GJ, Lopez A, et al. The different autophagy degradation pathways and neurodegeneration. Neuron. 2022;110:935–66.
Wang L, Klionsky DJ, Shen H-M. The emerging mechanisms and functions of microautophagy. Nat Rev Mol Cell Biol. 2022;24:186–203.
Zhang Y, Liu Z, Sun H. Fetal-maternal interactions during pregnancy: a ‘three-in-one’ perspective. Front Immunol. 2023;14:1198430.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381.
Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational Diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29:743–54.
Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218:S745–S61.
Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11.
Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Prim. 2023;9:8.
Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–9.
Petca A, Miron BC, Pacu I, Dumitrașcu MC, Mehedințu C, Șandru F, et al. HELLP Syndrome-holistic insight into pathophysiology. Medicina. 2022;58.
Tlaye KG, Endalfer ML, Kassaw MW, Gebremedhin MM, Aynalem YA. Preeclampsia management modalities and perinatal death: a retrospective study in Woldia general hospital. BMC Pregnancy Childbirth. 2020;20:205.
Jiang L, Tang K, Magee LA, von Dadelszen P, Ekeroma A, Li X, et al. A global view of hypertensive disorders and diabetes mellitus during pregnancy. Nat Rev Endocrinol. 2022;18:760–75.
Monod C, Kotzaeridi G, Linder T, Yerlikaya-Schatten G, Wegener S, Mosimann B, et al. Maternal overweight and obesity and its association with metabolic changes and fetal overgrowth in the absence of gestational diabetes mellitus: A prospective cohort study. Acta Obstet Gynecol Scand. 2024;103:257–65.
Andrews C, Maya J, Schulte CCM, Hsu S, Thaweethai T, James KE, et al. Risk of neonatal hypoglycemia in infants of mothers with gestational glucose intolerance. Diab Care. 2024;47:1194–201.
Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–9.
Lees CC, Romero R, Stampalija T, Dall’Asta A, DeVore GA, Prefumo F, et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am J Obstet Gynecol. 2022;226:366–78.
Melo P, Dhillon-Smith R, Islam MA, Devall A, Coomarasamy A. Genetic causes of sporadic and recurrent miscarriage. Fertil Steril. 2023;120:940–4.
Yusuf ANM, Amri MF, Ugusman A, Hamid AA, Wahab NA, Mokhtar MH. Hyperandrogenism and its possible effects on endometrial receptivity: a review. Int J Mol Sci. 2023;24:12026.
Rimmer MP, Teh JJ, Mackenzie SC, Al Wattar BH. The risk of miscarriage following COVID-19 vaccination: a systematic review and meta-analysis. Hum Reprod. 2023;38:840–52.
Carbonnel M, Pirtea P, de Ziegler D, Ayoubi JM. Uterine factors in recurrent pregnancy losses. Fertil Steril. 2021;115:538–45.
Zhang Y, Feng M, Gao Y, Zhang M, Zhang Z. Depression outcome in women with recurrent spontaneous abortion: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2024;300:54–62.
Costa MA. Scrutinising the regulators of syncytialization and their expression in pregnancy-related conditions. Mol Cell Endocrinol. 2016;420:180–93.
Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T, et al. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–16.
Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–74.
Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci USA. 2013;110:E231–E40.
Qin X-Y, Shen H-H, Zhou W-J, Mei J, Lu H, Tan X-F, et al. Insight of autophagy in spontaneous miscarriage. Int J Biol Sci. 2022;18:1150–70.
Voros C, Stavros S, Sapantzoglou I, Mavrogianni D, Daskalaki MA, Theodora M, et al. The role of placental mitochondrial dysfunction in adverse perinatal outcomes: a systematic review. J Clin Med. 2025;14:1422.
Marín R, Chiarello DI, Abad C, Rojas D, Toledo F, Sobrevia L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165961.
Zhao H, Wong RJ, Stevenson DK. The impact of hypoxia in early pregnancy on placental cells. Int J Mol Sci. 2021;22.
Dossou AS, Basu A. The emerging roles of mTORC1 in macromanaging autophagy. Cancers. 2019;11.
Rossant J, Tam PPL. Early human embryonic development: Blastocyst formation to gastrulation. Dev Cell. 2022;57:152–65.
Muter J, Lynch VJ, McCoy RC, Brosens JJ. Human embryo implantation. Development. 2023;150.
Diedrich K, Fauser BCJM, Devroey P, Griesinger G. The role of the endometrium and embryo in human implantation. Hum Reprod Update. 2007;13:365–77.
Moustafa S, Young SL. Diagnostic and therapeutic options in recurrent implantation failure. F1000Res. 2020;9.
Bao H, Wang H. Basic research advances in China on embryo implantation, placentation, and parturition. Matern Fetal Med. 2024;6:37–49.
Yang D, Liu A, Zhang Y, Nan S, Yin R, Lei Q, et al. Essential role of CRIM1 on endometrial receptivity in goat. Int J Mol Sci. 2021;22:5323.
Oestreich AK, Chadchan SB, Popli P, Medvedeva A, Rowen MN, Stephens CS, et al. The Autophagy Gene Atg16L1 is Necessary for Endometrial Decidualization. Endocrinology. 2020;161:bqz039.
Lai Z-Z, Wang Y, Zhou W-J, Liang Z, Shi J-W, Yang H-L, et al. Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics. 2022;12:6527–47.
Popli P, Tang S, Chadchan SB, Talwar C, Rucker EB, Guan X, et al. Beclin-1-dependent autophagy, but not apoptosis, is critical for stem-cell-mediated endometrial programming and the establishment of pregnancy. Dev Cell. 2023;58:885−897.e4.
Huang J, Liu F, Qi T, Gao R, Xie H, Ruan L, et al. Benzo(a)pyrene promotes autophagy to impair endometrial decidualization via inhibiting CXCL12/CXCR4 axis. Chem Biol Interact. 2025;405:111288.
Zhu Y, Zhang Z, Ma Z, Deng W, Zhang Y, Wu Q. Autophagy markers are dysregulated in the endometrial tissues of patients with unexplained repeated implantation failure. Mol Reprod Dev. 2022;89:655–60.
Yang Z, Li Q, Yuan F, Wang M, Zhang R, Chen Y, et al. Decreased NOTCH1 signaling activated autophagy in the mid-secretory endometrium of patients with recurrent implantation failure†. Biol Reprod. 2023;108:974–87.
Wang W-j, Zhang H, Chen Z-q, Zhang W, Liu X-m, Fang J-y, et al. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17:2.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res. 2019;124:1094–112.
Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15:275−89.
Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398:341–54.
Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022;226:S907–S27.
Nakashima A, Aoki A, Kusabiraki T, Cheng S-B, Sharma S, Saito S. Autophagy regulation in preeclampsia: Pros and cons. J Reprod Immunol. 2017;123:17–23.
Zhou M, Guo J, Li S, Li A, Fang Z, Zhao M, et al. Effect of peroxiredoxin 1 on the regulation of trophoblast function by affecting autophagy and oxidative stress in preeclampsia. J Assist Reprod Genet. 2023;40:1573–87.
Cheng S, Huang Z, Banerjee S, Jash S, Buxbaum JN, Sharma S. Evidence From Human Placenta, Endoplasmic Reticulum-Stressed Trophoblasts, and Transgenic Mice Links Transthyretin Proteinopathy to Preeclampsia. Hypertension. 2022;79:1738–54.
Jin J, Gao L, Zou X, Zhang Y, Zheng Z, Zhang X, et al. Gut dysbiosis promotes preeclampsia by regulating macrophages and trophoblasts. Circ Res. 2022;131:492–506.
Nakashima A, Shima T, Aoki A, Kawaguchi M, Yasuda I, Tsuda S, et al. Placental autophagy failure: A risk factor for preeclampsia. J Obstet Gynaecol Res. 2020;46:2497–504.
Li L, Peng W, Zhou Q, Wan J-P, Wang X-T, Qi H-B. LRP6 regulates Rab7-mediated autophagy through the Wnt/β-catenin pathway to modulate trophoblast cell migration and invasion. J Cell Biochem. 2020;121:1599–609.
Zhou X, Zhao X, Zhou W, Qi H, Zhang H, Han T-L, et al. Impaired placental mitophagy and oxidative stress are associated with dysregulated BNIP3 in preeclampsia. Sci Rep. 2021;11:20469.
Nakashima A, Shima T, Tsuda S, Aoki A, Kawaguchi M, Yoneda S, et al. Disruption of Placental Homeostasis Leads to Preeclampsia. Int J Mol Sci. 2020;2:13298.
Qiu L, Liu H, Chen S, Wu Y, Yan J. Ferroptosis contributed to endoplasmic reticulum stress in preterm birth by targeting LHX1 and IRE-1. Cell Signal. 2025;132:111777.
Chen H, Chen Y, Zheng Q. The regulated cell death at the maternal-fetal interface: beneficial or detrimental?. Cell Death Discov. 2024;10:100.
Liu M, Wu K, Wu Y. The emerging role of ferroptosis in female reproductive disorders. Biomed Pharmacother. 2023;166:115415.
Du J, Ji Q, Dong L, Meng Y, Xin G. HDAC4 Knockdown Induces Preeclampsia Cell Autophagy and Apoptosis by miR-29b. Reprod Sci. 2021;28:334–42.
Li Y, Guo Y, Wu D, Ai L, Wu R, Ping Z, et al. Phenylbutyric acid inhibits hypoxia-induced trophoblast apoptosis and autophagy in preeclampsia via the PERK/ATF-4/CHOP pathway. Mol Reprod Dev. 2024;91:e23742.
Vangrieken P, Al-Nasiry S, Bast A, Leermakers PA, Tulen CBM, Janssen GMJ, et al. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS One. 2021;16:e0245155.
Jiang N, Zhou M, Le Y, Xiao L, Zhang C, Li S, et al. The Effect of FPR2 on the Regulation of Trophoblast Autophagy via the PI3K/AKT/mTOR Signaling Pathway in Preeclampsia. FASEB J. 2025;39:e70697.
Sun J, Yu M, Du W, Zhu S, Chen Z, Tao J, et al. The cGAS-STING pathway promotes the development of preeclampsia by upregulating autophagy: Mechanisms and implications. Int Immunopharmacol. 2024;128:111531.
Alves P, Amaral C, Teixeira N, Correia-da-Silva G. Cannabidiol disrupts apoptosis, autophagy and invasion processes of placental trophoblasts. Arch Toxicol. 2021;95:3393–406.
Wu H, Liu K, Zhang J. Excess fibronectin 1 participates in pathogenesis of pre-eclampsia by promoting apoptosis and autophagy in vascular endothelial cells. Mol Hum Reprod. 2021;27:gaab030.
Gu S, Zhou C, Pei J, Wu Y, Wan S, Zhao X, et al. Esomeprazole inhibits hypoxia/endothelial dysfunction-induced autophagy in preeclampsia. Cell Tissue Res. 2022;388:181–94.
Gao Y, Zhang X, Meng T. Overexpression of let-7b exerts beneficial effects on the functions of human placental trophoblasts by activating the ERK1/2 signaling pathway. Mol Reprod Dev. 2022;89:39–53.
Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946.
Wang Y, Ji L, Peng Z, Lai R, Zhang X, Xu Y, et al. Silencing DAPK3 blocks the autophagosome-lysosome fusion by mediating SNAP29 in trophoblast cells under high glucose treatment. Mol Cell Endocrinol. 2020;502:110674.
Hung T-H, Huang S-Y, Chen S-F, Wu C-P, Hsieh. Ts-Ta. Decreased placental apoptosis and autophagy in pregnancies complicated by gestational diabetes with large-for-gestational age fetuses. Placenta. 2020;90:27–36.
Liu Q, Han Y, Zhang M, Yang P, Xiang Y, Chen M, et al. IGF1R stimulates autophagy, enhances viability, and promotes insulin secretion in pancreatic β cells in gestational diabetes mellitus by upregulating ATG7. Reprod Biol. 2024;24:100850.
Zheng J, Ma X, Zhou Y, Ge S, Sun A, Luo S, et al. GATA2/FGF21 axis regulates the effects of high glucose on the apoptosis, autophagy and oxidative stress of human umbilical vein endothelial cell via PI3K/AKT/mTOR Pathway. Ann Clin Lab Sci. 2022;52:278–91.
Bao Y, Zhang J, Liu Y, Wu L, Yang J. Identification of human placenta-derived circular RNAs and autophagy related circRNA-miRNA-mRNA regulatory network in gestational diabetes mellitus. Front Genet. 2022;13:1050906.
Ji Y, Zhang W, Yang J, Li C. MiR-193b inhibits autophagy and apoptosis by targeting IGFBP5 in high glucose-induced trophoblasts. Placenta. 2020;101:185–93.
Han D, Jiang L, Gu X, Huang S, Pang J, Wu Y, et al. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J Cell Physiol. 2020;235:8839–51.
Ji L, Chen Z, Xu Y, Xiong G, Liu R, Wu C, et al. Systematic characterization of autophagy in gestational diabetes mellitus. Endocrinology. 2017;158:2522–32.
Fetal Growth Restriction. ACOG Practice Bulletin, Number 227. Obstet Gynecol. 2021;137:e16–e28.
Kiserud T, Piaggio G, Carroli G, Widmer M, Carvalho J, Neerup Jensen L, et al. The World Health Organization Fetal Growth Charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 2017;14:e1002220.
Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. 2021;152:3−57.
Devarajan A, Rajasekaran NS, Valburg C, Ganapathy E, Bindra S, Freije WA. Maternal perinatal calorie restriction temporally regulates the hepatic autophagy and redox status in male rats. Free Radic Biol Med. 2019;130:592–600.
Chen W, Mehlkop O, Scharn A, Nolte H, Klemm P, Henschke S, et al. Nutrient-sensing AgRP neurons relay control of liver autophagy during energy deprivation. Cell Metab. 2023;35:786−806.e13.
Li M-R, Chen E-X, Li Z-H, Song H-L, Zhang Y, Li F-F, et al. HMGB1 regulates autophagy of placental trophoblast through ERK signaling pathway. Biol Reprod. 2024;111:414–26.
Li R, Peng J, Zhang W, Wu Y, Hu R, Chen R, et al. Ambient fine particulate matter exposure disrupts placental autophagy and fetal development in gestational mice. Ecotoxicol Environ Saf. 2022;239:113680.
Zhang H, Zheng Y, Liu X, Zha X, Elsabagh M, Ma Y, et al. Autophagy attenuates placental apoptosis, oxidative stress and fetal growth restriction in pregnant ewes. Environ Int. 2023;173:107806.
Li J, Gao H, Xu Z, Gao B, Zhang L, Su B, et al. Gestational exposure to carbon black nanoparticles triggered fetal growth restriction in mice: The mediation of inactivating autophagy-lysosomal degradation system in placental ferroptosis. Sci Total Environ. 2025;959:178167.
Goudarzi ST, Vousooghi N, Verdi J, Mehdizadeh A, Aslanian-Kalkhoran L, Yousefi M. Autophagy genes and signaling pathways in endometrial decidualization and pregnancy complications. J Reprod Immunol. 2024;163:104223.
Sun Y, Sha M, Qin Y, Xiao J, Li W, Li S, et al. Bisphenol A induces placental ferroptosis and fetal growth restriction via the YAP/TAZ-ferritinophagy axis. Free Radic Biol Med. 2024;213:524–40.
Zhu H-L, Shi X-T, Xu X-F, Xiong Y-W, Yi S-J, Zhou G-X, et al. Environmental cadmium exposure induces fetal growth restriction via triggering PERK-regulated mitophagy in placental trophoblasts. Environ Int. 2021;147:106319.
Fan M, Wu H, Xie Y, Liu M, Yu X, Wang F, et al. Maternal Nutritional Status Governs Fetal Development by Modulating Imprinting Gene GAB1-Mediated Trophoblast Differentiation in the Placenta. Cell Prolif. 2025;58:e70069.
Guo Y, Huang C, Xu C, Qiu L, Yang F. Dysfunction of ZNF554 promotes ROS-induced apoptosis and autophagy in Fetal Growth Restriction via the p62-Keap1-Nrf2 pathway. Placenta. 2023;143:34–44.
Zhang H, Zha X, Zheng Y, Liu X, Elsabagh M, Wang H, et al. Mechanisms underlying the role of endoplasmic reticulum stress in the placental injury and fetal growth restriction in an ovine gestation model. J Anim Sci Biotechnol. 2023;14:117.
Zheng Y, Zha X, Zhang B, Elsabagh M, Wang H, Wang M, et al. The interaction of ER stress and autophagy in trophoblasts: navigating pregnancy outcome†. Biol Reprod. 2024;111:292–311.
Li J, Dong X, Liu J-Y, Gao L, Zhang W-W, Huang Y-C, et al. FUNDC1-mediated mitophagy triggered by mitochondrial ROS is partially involved in 1-nitropyrene-evoked placental progesterone synthesis inhibition and intrauterine growth retardation in mice. Sci Total Environ. 2024;908:168383.
Zhou H, Zhao C, Wang P, Yang W, Zhu H, Zhang S. Regulators involved in trophoblast syncytialization in the placenta of intrauterine growth restriction. Front Endocrinol. 2023;14:1107182.
Nakashima A, Cheng S-B, Ikawa M, Yoshimori T, Huber WJ, Menon R, et al. Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy. 2020;16:1771–85.
Huang J, Wan Z, Li J, Xiong X, Jiang R, Yang B, et al. Downregulation of NNMT affects trophoblast function via inhibiting COMP/CD36/ERK1/2 axis in recurrent spontaneous abortion. Cell Signal. 2025;132:111831.
Rezayat F, Esmaeil N, Nikpour P, Feizi A, Rezaei A. Different behavior of NK cells isolated from healthy women and women with recurrent spontaneous abortion after treatment with human amniotic epithelial cells. J Leukoc Biol. 2025;117:qiaf020.
Tan H-X, Yang S-L, Li M-Q, Wang H-Y. Autophagy suppression of trophoblast cells induces pregnancy loss by activating decidual NK cytotoxicity and inhibiting trophoblast invasion. Cell Commun Signal. 2020;18:73.
Liu N, Shen H, Wang Z, Qin X, Li M, Zhang X. Autophagy inhibition in trophoblasts induces aberrant shift in CXCR4+ Decidual NK cell phenotype leading to pregnancy loss. J Clin Med. 2023;12.
Hong L, Zhu YC, Liu S, Wu T, Li Y, Ye L, et al. Multi-omics reveals a relationship between endometrial amino acid metabolism and autophagy in women with recurrent miscarriage. Biol Reprod. 2021;105:393–402.
Girling J, Knight CL, Chappell L. Intrahepatic cholestasis of pregnancy: Green-top Guideline No. 43 2022. BJOG. 2022;129.
Hobson S, Gandhi S, Sobel M. Intrahepatic cholestasis of pregnancy. CMAJ. 2022;194:E1650.
Kostka L, Hruban L, Morávková P. Intrahepatic cholestasis of pregnancy. CESKA Gynekol. 2024;89:405–10.
Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899–909.
Yang X, Zhou Y, Li H, Song F, Li J, Zhang Y, et al. Autophagic flux inhibition, apoptosis, and mitochondrial dysfunction in bile acids-induced impairment of human placental trophoblast. J Cell Physiol. 2022;237:3080–94.
Fang Y, Fang D. Comprehensive analysis of placental gene-expression profiles and identification of EGFR-mediated autophagy and ferroptosis suppression in intrahepatic cholestasis of pregnancy. Gene. 2022;834:146594.
Dong R, Hu Y, Chen Q, Shan D, Yuxia W. Elevated GABRP expression is correlated to the excessive autophagy in intrahepatic cholestasis of pregnancy. Heliyon. 2023;9:e13221.
Bastida-Ruiz D, Yart L, Wuillemin C, Ribaux P, Morris N, Epiney M, et al. The fine-tuning of endoplasmic reticulum stress response and autophagy activation during trophoblast syncytialization. Cell Death Dis. 2019;10:651.
Chen Z, Geng Y, Gao R, Zhong H, Chen J, Mu X, et al. Maternal exposure to CeO2NPs derails placental development through trophoblast dysfunction mediated by excessive autophagy activation. J Nanobiotechnology. 2022;20:131.
Wang A, Li Z, Zhang D, Chen C, Zhang H. Excessive ER-phagy mediated by FAM134B contributes to trophoblast cell mitochondrial dysfunction in preeclampsia. Acta Biochim Biophys Sin. 2024;56:1446–59.
Li J, Zhang S, Zhang Y, Dai Y, Zhang Y, Yang A, et al. Atg9A-mediated mitophagy is required for decidual differentiation of endometrial stromal cells. Reprod Biol. 2022;22:100707.
Kommagani R, Moley KH, Jungheim ES, Lydon JP, Medvedeva A, Chadchan SB, et al. The autophagy protein, FIP200 (RB1CC1) mediates progesterone responses governing uterine receptivity and decidualization†. Biol Reprod. 2020;102:843–51.
Chu Y, Zhu C, Yue C, Peng W, Chen W, He G, et al. Chorionic villus-derived mesenchymal stem cell-mediated autophagy promotes the proliferation and invasiveness of trophoblasts under hypoxia by activating the JAK2/STAT3 signalling pathway. Cell Biosci. 2021;11:182.
Lee B, Shin H, Oh J-E, Park J, Park M, Yang SC, et al. An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1. Autophagy. 2021;17:1649–66.
Weel IC, Ribeiro VR, Romão-Veiga M, Fioratti EG, Peraçoli JC, Peraçoli MTS. Down-regulation of autophagy proteins is associated with higher mTOR expression in the placenta of pregnant women with preeclampsia. Braz J Med Biol Res. 2023;55:e12283.
Cheng S, Huang Z, Jash S, Wu K, Saito S, Nakashima A, et al. Hypoxia-Reoxygenation Impairs Autophagy-lysosomal Machinery In Primary Human Trophoblasts Mimicking Placental Pathology Of Early-onset Preeclampsia. Int J Mol Sci. 2022;23:10.
Chu Y, Chen W, Peng W, Liu Y, Xu L, Zuo J, et al. Amnion-derived mesenchymal stem cell exosomes-mediated autophagy promotes the survival of trophoblasts under hypoxia through mTOR pathway by the downregulation of EZH2. Front Cell Dev Biol. 2020;8:545852.
Lu L, Ma Y, Deng J, Xie J, Huang C. Lower ATG7 levels are associated with a higher risk of gestational diabetes mellitus: a cross-sectional study. Diab Metab Syndr Obes. 2022;15:2335–43.
Bao Y, Wu L, Liu Y, Fan C, Zhang J, Yang J. Role of CircCHD2 in the pathogenesis of gestational diabetes mellitus by regulating autophagy via miR-33b-3p/ULK1 axis. Placenta. 2024;145:27–37.
Zheng L, Tang R, Ahmad F, Fang J, Shi L, Chen X, et al. Circ_0081343 promotes autophagy and alleviates pyroptosis via PI3 K/AKT/HIF-1α axis in hypoxia-induced fetal growth restriction of mice. Anim Cells Syst. 2025;29:312–24.
Ling Q, Zhang Y-F, Chang W, Liu S-T, Zhu H-L, Wang H. NBR1-dependent autophagy activation protects against environmental cadmium-evoked placental trophoblast senescence. Chemosphere. 2024;358:142138.
Yang H-L, Lai Z-Z, Shi J-W, Zhou W-J, Mei J, Ye J-F, et al. A defective lysophosphatidic acid-autophagy axis increases miscarriage risk by restricting decidual macrophage residence. Autophagy. 2022;18:2459–80.
Tang L, Dai F, Zhang Y, Wang R, Tan W, Gu R, et al. Deletion of BMP4 impairs trophoblast function and decidual macrophage polarization via autophagy leading to recurrent spontaneous abortion. Int Immunopharmacol. 2025;147:114015.
Wang RQ, Dai F, Deng Z, Tang L, Liu H, Xia L, et al. ITGA3 participates in the pathogenesis of recurrent spontaneous abortion by downregulating ULK1-mediated autophagy to inhibiting trophoblast function. Am J Physiol Cell Physiol. 2024;328:C1941−C1956.
Lu H, Yang H-L, Zhou W-J, Lai Z-Z, Qiu X-M, Fu Q, et al. Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence. Autophagy. 2021;17:2511–27.
Yang D, Ding J, Wang Y, Yuan M, Xian S, Zhang L, et al. YY1-PVT1 affects trophoblast invasion and adhesion by regulating mTOR pathway-mediated autophagy. J Cell Physiol. 2020;235:6637–46.
Wang P, Zhao C, Zhou H, Huang X, Ying H, Zhang S, et al. Dysregulation of Histone Deacetylases inhibits trophoblast growth during early placental development partially through TFEB-Dependent Autophagy-Lysosomal Pathway. Int J Mol Sci. 2023;24:15.
Pan Y, Yan L, Chen Q, Wei C, Dai Y, Tong X, et al. Dysfunction of Shh signaling activates autophagy to inhibit trophoblast motility in recurrent miscarriage. Exp Mol Med. 2021;53:52–66.
Huang Z, Cheng S, Jash S, Fierce J, Agudelo A, Higashiyama T, et al. Exploiting sweet relief for preeclampsia by targeting autophagy-lysosomal machinery and proteinopathy. Exp Mol Med. 2024;56:1206–20.
Ma F, Ding N, Xie L, Zhao X, Ma S, Li G, et al. Inhibition of autophagy via 3-methyladenine alleviates the progression of preeclampsia. Acta Biochim Biophys Sin. 2024;57:356–64.
Wang P, Huang C-X, Gao J-J, Shi Y, Li H, Yan H, et al. Resveratrol induces SIRT1-dependent autophagy to prevent H2O2-induced oxidative stress and apoptosis in HTR8/SVneo cells. Placenta. 2020;91:11–8.
Yang F, Mao Y, Tang B, Xu R, Jiang F. Fatty acid binding protein 4 knockdown improves fetal development in rats with gestational diabetes mellitus through modulating autophagy mediated by the PTEN/Akt/mTOR signaling pathway. J Mol Histol. 2025;56:124.
Tao J, Rao Y, Wang J, Tan S, Zhao J, Cao Z, et al. Placental growth factor alleviates hyperglycemia-induced trophoblast pyroptosis by regulating mitophagy. J Obstet Gynaecol Res. 2024;50:1813–29.
Kokkinopoulou I, Papadopoulou A. Thioredoxin-Interacting Protein (TXNIP) in Gestational Diabetes Mellitus. Metabolites. 2025;15:351.
Yang T, Hu J, Zhang L, Liu L, Pan X, Zhou Y, et al. CircCUL1 inhibits trophoblast cell migration and invasion and promotes cell autophagy by sponging hsa-miR-30e-3p in fetal growth restriction via the ANXA1/PI3K/AKT axis. J Biochem Mol Toxicol. 2024;38:e23759.
Mu Y, Wang K, Kang Y, Fang Z, Yu S, Zhou J, et al. TBBPA-BDBPE induces ferroptosis in trophoblasts through chaperone-mediated autophagy of GPX4. Ecotoxicol Environ Saf. 2025;299:118425.
Zhao X, Jiang Y, Ren J, Wang Y, Zhao Y, Feng X. Deciphering the mechanism of Bushen Huoxue Decotion on decidualization by intervening autophagy via AMPK/mTOR/ULK1: A Novel Discovery for URSA treatment. Front Pharm. 2022;13:794938.
Li Z, Dai F, Zhu R, Zhang Y, Chen J, Chen L, et al. Dysregulation of CREB5 impairs decidualization and maternal–fetal interactions by inhibiting autophagy in recurrent spontaneous abortion. Reprod Sci. 2024;31:1983–2000.
Lin R-C, Chao Y-Y, Su M-T, Tsai H-L, Tsai P-Y, Wang C-Y. Upregulation of miR-20b-5p inhibits trophoblast invasion by blocking autophagy in recurrent miscarriage. Cell Signal. 2024;113:110934.
Sun Y, Li G, Kong M, Li J, Wang S, Tan Y. Angelica sinensis polysaccharide as potential protectants against recurrent spontaneous abortion: focus on autophagy regulation. Front Med. 2025;12:1522503.
Li M-Y, Shen H-H, Cao X-Y, Gao X-X, Xu F-Y, Ha S-Y, et al. Targeting a mTOR/autophagy axis: a double-edged sword of rapamycin in spontaneous miscarriage. Biomed Pharmacother. 2024;177:116976.
He W, Zhao Y, Yin L, Du Q, Ren W, Mao L, et al. The transcription factor XBP1 regulates mitochondrial remodel and autophagy in spontaneous abortion. Int Immunopharmacol. 2025;152:114398.
Acknowledgements
All the figures were created in https://BioRender.com.
Funding
This work was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0532100), National High Level Hospital Clinical Research Funding (22cz020401-4811009), Beijing Natural Science Foundation (5244036), Clinical Medicine Plus X-Young Scholars Project of Peking University (PKU2025 PKULCXQ034) and the National Key Research and Development Program of China (2021YFC2700704).
Author information
Authors and Affiliations
Contributions
WST conceived the structure, drafted the manuscript, and designed the related figures; HYX and YHX revised this manuscript; and all the authors read and approved the final manuscript. WST and HYX contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Oliana Carnevali
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, S., Huang, Y. & Yang, H. Shedding light on the function of autophagy in complicated pregnancies. Cell Death Dis (2026). https://doi.org/10.1038/s41419-025-08311-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-025-08311-7