Abstract
Translation is a decoding process that synthesizes proteins from RNA, typically mRNA. The conventional translation process consists of four stages: initiation, elongation, termination, and ribosome recycling. Precise control over the translation mechanism is crucial, as dysregulation in this process is often linked to human diseases such as cancer. Recent discoveries have unveiled translation mechanisms that extend beyond typical well-characterized components like the m7G cap, poly(A)-tail, or translation factors like eIFs. These mechanisms instead utilize atypical elements, such as non-canonical ORF, m6A-modification, and circular RNA, as key components for protein synthesis. Collectively, these mechanisms are classified as non-canonical translations. It is increasingly clear that non-canonical translation mechanisms significantly impact the various regulatory pathways of cancer, including proliferation, tumorigenicity, and the behavior of cancer stem cells. This review explores the involvement of a variety of non-canonical translation mechanisms in cancer biology and provides insights into potential therapeutic strategies for cancer treatment.

Similar content being viewed by others
Facts
-
Non-canonical translation mechanisms enhance the translation of oncogenes, growth factors, and anti-apoptotic proteins, contributing to cancer development and progression, yet the intricacies of these processes remain largely unexplored.
-
Non-canonical open reading frames (ORFs), including short or small ORFs, upstream ORFs, 3′-UTR downstream ORFs, and long non-coding RNAs, are widespread in human transcripts and exhibit differential regulation in tumor cells.
-
N6-methyladenosine (m6A) modification of RNA is emerging as a key player in non-canonical translation mechanisms, particularly in cancer biology, by initiating or enhancing the translation of mRNAs as well as non-coding RNAs.
-
While circular RNAs are recognized for their role as sponges for miRNAs and proteins in cancer cells, proteins or peptides encoded by circular RNAs have emerged as significant regulators of cancer progression.
Open questions
-
What molecular mechanisms govern non-canonical translation in cancer cells?
-
How do proteins produced through non-canonical translation influence the survival and progression of cancer cells?
-
How can we specifically target non-canonical translation pathways to inhibit tumor progression?
-
How can insights from products translated through non-canonical mechanisms, especially from non-canonical ORFs and circular RNAs, be utilized to develop neoantigens for cancer immunotherapy?
Introduction
Canonical translation
Translation of RNA is a fundamental yet complex biological process in which proteins are produced by decoding the codon sequences of RNA molecules, usually mRNAs. Importantly, dysregulated translation is often the cause or a contributing factor to many diseases, including cancer. Translation is carried out in four stages: initiation, elongation, termination and ribosome recycling. Initiation, considered the rate-limiting phase, involves five steps (Fig. 1A) [1, 2]: (1) The 43S preinitiation complex (PIC) forms by combining 40S subunits with eukaryotic initiation factors (eIFs), including eIF1, eIF1A, eIF3, and eIF5, along with the ternary complex of eIF2–GTP-methionyl-tRNA initiator (Met-tRNAi). (2) The eIF4F complex, consisting of the cap-binding protein eIF4E, a scaffold protein eIF4G, and the RNA helicase eIF4A, binds to the 5′ m7GpppN (m7G) cap (hereafter referred to as the cap) and interacts with poly(A)-binding protein (PABP), associated with the poly(A)-tail, leading to mRNA activation. (3) The 43S PIC is recruited to the activated mRNA, forming the 48S initiation complex (IC). (4) The 48S IC scans the 5′ untranslated region (UTR) until recognition of the start codon AUG, allowing base-pairing with Met-tRNAi. (5) The 60S subunit is integrated in concert with the release of initiation factors including eIF1, eIF2, eIF3, and eIF5, resulting in the assembly of the 80S IC.
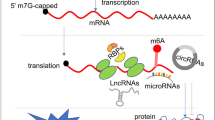
A The conventional translation process commences with the formation of an mRNA closed-loop structure, facilitated by multiple interactions involving eIF4E with the mRNA cap and PABP with the poly(A)-tail. Subsequently, eIF4G binds to both eIF4E and eIF4A, forming the eIF4F complex. The eIF4F complex also interacts with PABP via eIF4G. This complex formation assists in recruiting the 43S PIC, comprising the 40S ribosomal subunit, eIF1, eIF1A, eIF3, eIF5, and the eIF2-GTP-Met-tRNAi ternary complex, to the mRNA cap. The 43S PIC then begins scanning from the 5′ to the 3′ direction in an ATP-dependent manner until it recognizes the AUG start codon. GTP hydrolysis then leads to the release of eIFs, allowing the large 60S ribosomal subunit to join and form the 80S ribosome. Translation continues until a stop codon is encountered, at which point the 80S complex is released, and the components are recycled for subsequent translation events. B Non-canonical translation mechanisms can occur independently of a cap or poly(A)-tail, and may utilize non-AUG start codons.
During elongation, ribosomes catalyze the formation of peptide bonds between consecutive amino acids carried by tRNAs. Upon bond formation, the ribosome releases the empty tRNA and proceeds along the mRNA in a 5′ to 3′ direction with the aid of GTP hydrolysis and elongation factors (EFs). These steps are reiterated until the ribosome reaches a stop codon. At this point, release factors attach to the ribosome, leading to the release of the fully formed polypeptide chain and termination of translation, enabling the recycling of the ribosome for subsequent rounds of translation.
Non-canonical translation
Translational mechanisms that bypass the use of the cap, poly(A)-tail, or the standard AUG start codon are classified as non-canonical translations (Fig. 1B) [1, 3]. Emerging research has uncovered alternative pathways for mRNA translation [1, 4, 5]. For example, in previous research, we discovered a process named PAINT (Poly(A)-tail Independent Non-canonical Translation) during early embryogenesis, which operates independently of a poly(A)-tail [6]. We also identified a specific inhibitor of PAINT, known as primordazine, and found that this mechanism is essential for primordial germ cell (PGC) maintenance, as primordazine treatment results in PGC loss [6, 7].
Although non-canonical translation triggered by non-AUG codons [8, 9] or internal ribosome entry sites (IRES) proceeds at a reduced pace compared to canonical translation, it has demonstrated its significance in a variety of biological conditions, including stress responses and cancer cells [5, 9, 10]. Non-canonical translation also plays a significant role in numerous diseases. Notably, many neurological disorders, such as Huntington’s disease, many types of spinocerebellar ataxia, and amyotrophic lateral sclerosis, are linked to non-AUG initiated translation, known as repeat-associated non-AUG (RAN) translation [10,11,12]. Furthermore, non-canonical translation has significant implications for cancer progression and proliferation, enabling cancer cells to adapt to diverse stress growth conditions [4, 5, 13, 14].
In this article, we review the latest findings on how non-canonical translation pathways regulate tumorigenesis. Additionally, potential therapies targeting these pathways will be briefly discussed, where applicable. Technological approaches for identifying non-canonical translation, reviewed elsewhere [15,16,17], will not be covered in this paper.
Non-canonical ORFs
Non-canonical ORFs are widespread in various organisms [18]. However, their potential to translate into proteins has been questioned for a long time, hindering their acceptance within the RNA translation research community [18, 19]. Recent advances in genomic, translational, and proteomic techniques have uncovered additional roles of non-canonical ORFs that extend beyond traditional translational regulation [20,21,22,23,24]. These non-canonical ORFs include short or small ORFs (sORFs or smORFs), upstream ORFs (uORFs), 3′-UTR downstream ORFs (dORFs), and long non-coding RNAs (lncRNAs). Studies show that peptides encoded by uORFs exist in both healthy and tumor cells, influencing cellular functions, metabolism, and immune pathways, and are involved in many diseases, especially cancer [15, 19, 20, 23, 24]. This evidence suggests that non-canonical ORFs possess the capacity to encode proteins or peptides, potentially implicating them in pathological processes. However, the precise translation mechanisms and functions of these products are yet to be fully understood.
Mechanisms of non-canonical ORF translation
sORF, a prevalent type of non-canonical ORF consisting of fewer than 150, typically 100, amino acid (aa), encompasses various forms such as sORF, uORF, and dORF. Additionally, lncRNAs are emerging as a form of sORF [15,16,17]. Initially overlooked, sORFs and their encoded peptides (micropeptides or sORF-encoded peptides) were once dismissed, possibly because codons under 100-aa were considered non-functional. sORFs contain a substantial number of non-AUG initiation codons, with over 50% of sORFs utilizing these non-AUG start codons [8, 10, 20, 25]. They have been found in various RNA positions, such as within a canonical coding sequence (CDS) region as an out-of-frame overlapping ORF (Fig. 2A), in non-coding RNA (ncRNA) transcripts like lncRNAs (Fig. 2B), and in 5′-UTRs (Fig. 2C). Notably, sORFs have been recognized to play crucial roles in various biological processes, including cancer, metabolism, and immunity [8, 15,16,17, 26,27,28,29,30,31,32,33]. Intriguingly, a significant portion of transcribed enhancers, known as enhancer RNAs, harbor sORFs that can generate microproteins [34].
A Small ORFs (sORFs) can be found in various positions within RNA, including the 5′-UTR, the 3′-UTR, within the CDS, or interspersed between these regions. B sORFs can be also found in non-coding RNA, such as lncRNA. C Upstream ORFs (uORFs) are encoded in the 5′-UTR, allowing for translation initiation. D Downstream ORFs (dORFs) are located in the 3′-UTR. Translation of dORFs enhances the translation of their respective CDS, differing from uORFs which frequently inhibit the translation of the canonical downstream mORF. E Diagram illustrating potential therapeutic approaches for cancer utilizing non-canonical ORF translation. Neoantigens are an invaluable resource for promising cancer treatments through adoptive cell transfer (ACT) or immune checkpoint blockade (ICB).
uORFs are a well-recognized type of non-canonical ORF in the human genome (Fig. 2C) and often contain at least one AUG start codon in about 50% of cases, upstream of the main ORF (mORF) [10], indicating their potential for translatability. Traditionally, uORFs have been regarded as regulatory elements that influence the translation of mORF through mechanisms like leaky scanning and ribosome stalling, rather than serving as CDSs for functional proteins or peptides [15, 18, 35]. Despite this conventional view, a growing body of research has discovered that many uORFs are able to generate functional peptides or proteins [22, 36]. Ribosome analysis suggests that about 30% of mammalian transcripts may contain actively translated uORFs in their 5′-UTR regions [22]. Moreover, non-AUG start codons are frequently used for the translation of many uORFs [8,9,10, 22, 36]. CRISPR knockout screening has uncovered various micropeptides encoded by uORFs [20]. These micropeptides play roles in diverse biological functions, including cell proliferation [20, 37], stress responses [31], and mitochondrial dynamics [20, 37]. Interestingly, proteins encoded by the uORF often interact with proteins expressed from the downstream canonical mORF, suggesting a potential role over the mORF-encoded protein [20, 37].
dORF, another subtype of sORF, is relatively less common compared to uORF. It is situated in the 3′-UTR downstream of the CDS (Fig. 2D). The translation of dORF enhances the translation of its corresponding CDS [38], in contrast to uORFs, which often suppress the translation of the canonical downstream mORF [35]. Furthermore, substantial translational activity in 3′-UTR regions has been noted in numerous ribosome profiling analyses. Various hypotheses have been suggested to elucidate ribosome binding in the 3′-UTR [15]. One model proposes that ribosome doesn’t stop at a stop codon and continues translation, known as stop codon readthrough, leading to extended translation of the canonical CDS. Two models are suggested to explain how dORF translation can be initiated. The ribosome translation complex temporarily dissociates from the canonical CDS at its stop codon and then reinitiate at the dORF via a presently unknown mechanism. Alternatively, an IRES located in the 3′-UTR may facilitate ribosome recruitment, initiating translation independently of the cap [15, 39]. Although numerous translation products from dORF have been detected through mass spectrometry [25, 40, 41], their functional elucidation remains understudied. A study reported that a peptide derived from dORF of the ABCB5 gene exhibits immunogenicity in melanoma, acting as a non-canonical human leukocyte antigen (HLA)-binding peptide [40]. Currently, relevant reports are still limited, and confirmations are lacking regarding the translation products of dORFs and their precise roles. Further exploration is necessary to understand the translation mechanism of dORFs.
Non-canonical ORFs in cancer
Mounting evidence underscores the substantial role of peptides or proteins derived from non-canonical ORFs in contributing to cancer development. This session highlights the latest key findings regarding non-canonical ORFs in cancer research (Table 1).
p27KIP1, encoded by the CDKN1B gene, functions as a tumor suppressor involved in cell proliferation and differentiation. Reduced levels of p27KIP1 are commonly observed in tumor samples, and CDKN1B mutations, though rare, have been identified in several cancers [42]. Notably, a 4-bp deletion in the uORF of CDKN1B was identified in a patient with pituitary and pancreatic tumors [43]. This deletion alters the termination codon of the uORF, affecting the length of the uORF-encoded peptide and intercistronic space, ultimately resulting in reduced p27KIP1 expression. This highlights the mutation in uORF as a novel mechanism impacting p27KIP1 levels, although biological functions of uORF-encoded peptide remain to be elucidated.
Initially classified as a lincRNA, the CASIMO1 gene was later discovered to encode a 10 kDa transmembrane microprotein, CASIMO1, now officially designated as Small Integral Membrane Protein 22 (SMIM22). The transcript and protein levels of CASIMO1 are significantly upregulated in hormone receptor-positive (ER+ or PR+) breast tumors [44]. Knocking it down inhibits cell proliferation, disrupts cytoskeletal organization and cell motility, and triggers G0/G1 cell cycle arrest. Squalene Epoxidase (SQLE) is a rate-limiting enzyme crucial for the cholesterol biosynthesis pathway, converting squalene to 2,3-oxidosqualene. SQLE, recognized for its oncogenic properties and its role in sterol biosynthesis, has been identified as a target of CASIMO1. CASIMO1 enhances SQLE protein levels by directly interacting with and protecting SQLE from degradation, leading to the accumulation of lipid droplets. Conversely, the absence of CASIMO1 leads to a decrease in SQLE protein levels, accompanied by reduced phosphorylation of ERK, a downstream effector of SQLE. CASIMO1 is the first functional microprotein implicated in both carcinogenesis and cellular lipid homeostasis.
ZNFX1 antisense RNA 1 (ZFAS1) is a lncRNA involved in the innate immune response and gene regulation by sponging various microRNAs. A study identified 537 potential sORFs, out of which five were experimentally validated [45]. Analysis of 11 lncRNAs across seven cancer types has revealed that ZFAS1 was notably overexpressed in hepatocellular carcinoma (HCC). Higher levels of ZFAS1 lead to enhanced cancer cell motility, presumably due to an increase in reactive oxygen species. However, it is still unclear whether this tumor-promoting effect is directly attributable to a protein encoded by a sORF within ZFAS1. Another study discovered KRASIM, a 99-aa micropeptide encoded by the lncRNA NCBP2-AS2 [46]. KRASIM inhibits the growth and proliferation of HCC cells by interacting with and reducing KRAS protein levels, consequently decreasing ERK signaling activity.
Triple-negative breast cancer (TNBC) represents a highly aggressive form of breast cancer, associated with unfavorable outcomes and a poor prognosis. LINC00908, identified as a specifically downregulated lncRNA in TNBC, is under the regulation of estrogen receptor α (ERα). LINC00908 harbors a sORF that encodes ASRPS, a 60-aa micropeptide [47]. ASRPS plays a crucial role in inhibiting STAT3 phosphorylation, leading to a reduction in VEGF expression and angiogenesis. Low expression of ASRPS is linked to poor survival in TNBC patients. In mouse models, ASRPS also exhibits anti-tumor properties. ASRPS, produced by LINC00908, presents a promising avenue for targeted therapy in TNBC.
The micropeptide CIP2A-BP, derived from LINC00665, is translationally suppressed by TGF-β in breast cancer [48]. In TNBC, activated SMAD signaling upregulates 4E-BP1, an inhibitory protein for cap-dependent translation that acts by inhibiting elF4E, resulting in the reduced translation of CIP2A-BP. The role of CIP2A-BP is known to disrupt the CIP2A-PP2A interaction, leading to suppression of the PI3K/AKT/NF-κB pathway and inhibition of metastatic factors. Consequently, reduced levels of CIP2A-BP are linked to TNBC metastasis and poorer survival. Introducing CIP2A-BP gene or its micropeptide in a mouse model significantly reduces metastases and improves survival. Thus, CIP2A-BP is a valuable prognostic marker and a promising therapeutic target for TNBC.
The micropeptide YY1BM, originating from the sORF1 of the Y-linked lncRNA LINC00278, is found to be downregulated in male esophageal squamous-cell carcinoma (ESCC) [49]. YY1BM functions by inhibiting the interaction between the transcription factor YY1 and the androgen receptor, resulting in a reduced level of eEF2K. The diminished expression of YY1BM in ESCC leads to a significant increase in eEF2K expression, a factor believed to inhibit apoptosis, thus making ESCC cells more resistant to nutrient deprivation. Additionally, smoking has been shown to reduce YY1BM translation by decreasing the m6A-modification of LINC00278.
The lncRNA LOC90024 was discovered to encodes a 130-aa protein called Splicing Regulatory Small Protein (SRSP) [50]. SRSP interacts with splicing regulators, particularly SRSF3, to modulate mRNA splicing. SRSP, not LOC90024 itself, drives colorectal cancer (CRC) progression. SRSP promotes CRC tumorigenesis by enhancing the cancerous long Sp4 isoform. Elevated SRSP levels are linked to aggressive CRC and poor prognosis, making it a potential biomarker and therapeutic target.
A micropeptide called micropeptide inhibiting actin cytoskeleton (MIAC), encoded by RP11-469H8.6, has been identified as a crucial factor in head and neck squamous-cell carcinoma (HNSCC) [51]. Reduced MIAC expression is linked to poorer overall survival rates in HNSCC patients and is significantly associated with the progression of five other distinct tumor types. MIAC’s interaction with AQP2 plays a role in negatively regulating the levels of SEPT2 and ITGB4, leading to the inhibition of the actin cytoskeleton. Consequently, this inhibitory effect suppresses HNSCC tumor growth and metastasis.
The gastrointestinal-specific lncRNA, LINC00675, encodes FORCP (FOXA1-Regulated Conserved Small Protein), a 79-aa micropeptide [52]. Under the regulation of FOXA1, FORCP is typically scarce in most cells but abundant in well-differentiated CRC cells, as FOXA1 is the only transcription factor enriched in these cells. FORCP predominantly localizes to the ER, and its depletion results in reduced apoptosis during ER stress or glucose deprivation. It effectively inhibits proliferation, clonogenicity, and tumorigenesis by acting as a pro-apoptotic factor in response to ER stress.
Derived from pri-miR-34a, the 133-aa microprotein miPEP133 acts as a tumor suppressor by inducing apoptosis, inhibiting cancer cell migration and invasion, and suppressing tumor growth [53]. miPEP133 is downregulated in cancer cell lines and tumors. Lower miPEP133 levels correlate with poorer prognosis in nasopharyngeal carcinoma. Functionally, miPEP133 binds with mitochondrial chaperone HSPA9, inhibiting its function by reducing mitochondrial membrane potential, ATP production, and ultimately inducing apoptosis. Notably, wild-type p53, but not mutant p53, enhances miPEP133 expression. In turn, miPEP133 enhances the transcriptional activity of p53, subsequently increasing miR-34a expression.
Prensner et al. investigated 553 non-canonical ORF candidates and identified 57 that caused viability issues when removed from human cancer cells [23]. Among these candidates, 257 exhibited protein expression upon ectopic introduction, and 401 induced alterations in gene expression. Notably, the ORF G029442, now termed GREP1 (Glycine-Rich Extracellular Protein-1), encodes a secreted protein highly prevalent in breast cancer. Deletion of GREP1 in 263 cancer cell lines resulted in a preferential loss of viability in certain cell lineages, particularly in breast cancer, highlighting its importance for cancer cell survival. The secretome of GREP1-expressing cells featured heightened levels of the oncogenic cytokine GDF15, contributing to GREP1’s growth effect.
The 1p36 region, recognized as a crucial tumor suppressor locus, is frequently subject to deletion in cancer. A CpG methylome analysis unveiled the silenced KIAA0495/GFOD3P gene at 1p36.3, revealing its encoding of a small protein named SP0495 [54]. Promoter CpG methylation often hampers the transcription of KIAA0495, resulting in the depletion of SP0495 in various cancers and correlating with poor survival rates among cancer patients. SP0495, through its binding to phosphoinositides, effectively inhibits AKT, mTOR, NF-κB, and Wnt/β-catenin signaling pathways, thereby restraining tumor growth. Additionally, it promotes autophagy by regulating autophagy regulators BECN1 and SQSTM1/p62. This newfound role positions SP0495 as a potential biomarker and a novel methylation-sensitive tumor suppressor in multiple cancers.
LINC00998, initially categorized as a lncRNA, is overexpressed in various cancers. It houses a sORF encoding a micropeptide called SMIM30, located in ER and mitochondrial membranes [55]. Silencing SMIM30 curtails hepatoma cell proliferation and inhibits tumor growth. Introducing a premature stop codon into the sORF abolishes its tumor-promoting effect. Functional assays reveal SMIM30, not LINC00998, controls cytosolic calcium levels, CDK4, cyclin E2, phosphorylated-Rb, and E2F1, driving the G1/S phase transition and cell proliferation. This effect is attenuated by a calcium chelator or SERCA pump agonist, indicating SMIM30’s crucial role in cancer progression.
A recent study that combined ribosome profiling with CRISPR-Cas9 screening has identified several cryptic ORFs encoded by lncRNAs that are crucial for the survival of ERα+ breast cancer cells [56]. Among these, LINC00992 has been linked to poor outcomes in luminal breast cancer and is responsible for producing the GATA3-interacting cryptic protein (GT3-INCP). GT3-INCP significantly enhances cell proliferation and tumor growth, an effect that is facilitated by its interaction with GATA3, a key transcription factor involved in the development of luminal epithelial cells. This interaction between GT3-INCP and GATA3 leads to the cooperative regulation of estrogen-responsive and tumor-promoting genes, including MYB and PDZK1.
MYCN or MYCC stands out as one of the most frequently implicated cancer driver genes. The 5′-UTR of MYCN and MYCC mRNAs encompasses uORFs, resulting in MNOP and MYCNOT for MYCN, and mrtl and MYCHEX1 for MYCC [57]. These uORF-encoded proteins are translated to easily detectable levels in tumor cell lines. Upon treatment with JQ1, a bromodomain and extraterminal domain (BET) inhibitor, MYCN, MYCNOT, and mrtl nearly vanish, coinciding with a notable increase in apoptosis levels in pediatric embryonal tumor cell lines that rely on MYC to maintain an undifferentiated phenotype. Although the precise roles of these uORF‐encoded proteins in tumor pathology remain unclear, this study lays the groundwork for the potential involvement of uORF in cancer.
Another study, using ribosome profiling and CRISPR-Cas9 screens on various medulloblastoma samples, revealed the widespread translation of non-canonical ORFs with functions distinct from their primary CDSs [58]. The ASNSD1-uORF protein, also known as ASDURF, derived from a uORF within the ASNSD1 gene was found to exhibit elevated expression in medulloblastoma. It is indispensable for MYC-driven medulloblastoma cells, but not for non-MYC-driven medulloblastoma or other types of cancer cells. It plays a pivotal role in the survival of medulloblastoma cells through its interaction with the prefoldin-like chaperone complex. This finding highlights the significant role of non-canonical ORF translation in medulloblastoma and underscores the importance of these ORFs and their translation products as promising focuses for developing new therapeutic strategies.
Prospects for cancer therapy involving non-canonical ORFs
Small peptides arising from non-canonical ORFs, such as CIP2A-BP, SRSP, and miPEP133 [48, 50, 53], serve not only as potential prognostic markers for specific cancer types but also as a significant source of neoantigens, distinct proteins/peptides exclusive to cancer cells and absent in normal tissues (Fig. 2E).
Adoptive cell transfer (ACT) and immune checkpoint blockade (ICB) are highly promising tumor treatments. ACT involves treating cancer patients with their own T cells, which can be naturally occurring or genetically modified, including tumor-infiltrating lymphocytes (TILs), T cells engineered with receptors, or chimeric antigen receptor (CAR) cells. Efforts to develop neoantigen-targeted T cell immunotherapies have gained momentum [59, 60].
Neoantigens originate from tumors through diverse mechanisms, such as DNA mutations, atypical transcriptomic variations, alterations in post-translational modifications, viral ORFs, and non-canonical ORFs [19, 60]. These neoantigens are subsequently presented on the surface of cancer cells through both class-I and II HLA, also known as major histocompatibility (MHC) molecules. Neoantigens exhibit substantial promise as targets for customized cancer immunotherapies.
Recent studies underscore the importance of non-canonical uORF sequences in shaping the immunopeptidome of malignant tissues. For example, meloe has been identified as a polycistronic mRNA generating the melanoma antigens MELOE-1 and MELOE-2 through an IRES mechanism [61]. Furthermore, a peptide originating from the reverse strand in the 3′-UTR of tRNA isopentenyltransferase 1 (TRIT1) serves as an antigen or sensitizes HLA-B57+ melanoma cells to lysis by cytotoxic T lymphocyte [62]. Somatic uORF mutations are widespread in various cancers, potentially playing a role in disease onset and progression [63, 64]. Moreover, a recent study revealed numerous functional micropeptides from non-canonical ORFs, some of which are presented by HLA, influencing antigen repertoire and immunogenicity [20]. These studies shed light on a previously neglected dimension of the immune response against cancer.
Using mass spectrometry-based immunopeptidome analysis, 125 distinct HLA uLigands originating from 120 uORFs of 79 genes, such as ASNSD1, ATF5, MAPK1, and TMEM203, were discovered. In the pursuit of frequent tumor-associated uORF antigens, these exclusive HLA uLigands were ranked by their occurrence in malignant tissue. This led to the identification of 16 HLA uLigands as tumor-associated uORF neoantigens [65]. Given the analysis’s restriction to about 2000 uORF sequences, the sheer abundance of over 2.4 million AUG- and alternative translation initiation sites-initiated uORFs in the human transcriptome [36] implies that future studies hold the potential to reveal a plethora of additional tumor-specific HLA uLigands.
Targeting tumor-specific neoantigens holds promise for precise tumor eradication while minimizing off-target effects. However, challenges arise from the diverse antigen processing and presentation methods employed by tumors, as well as an incomplete understanding of essential T cell characteristics for clinical effectiveness. Thus, meticulous antigen selection is crucial for ensuring both safety and efficacy. Certain strategies targeting broadly expressed antigens have resulted in ‘off-tumor, on-target’ toxicities, underscoring the need for caution when considering self-antigens co-expressed on vital tissues for clinical testing.
In a recent investigation, personalized neoantigen-HLA capture libraries were created from metastatic melanoma patients, encompassing responders and non-responders to anti-PD-1 immunotherapy [66]. This led to the isolation of neoantigen-specific T cell receptors (neoTCRs). Responders displayed distinct T cell clonotypes recognizing a specific set of mutations, persistently present over time. Conversely, non-responders exhibited lower TCR diversity and sporadic detection of neoantigen-specific T cells, with limited recurrence. T cells from healthy donors, when genetically engineered through non-viral CRISPR-Cas9 gene editing to become neoTCR gene-edited T cells, exhibit specific T cell-mediated cytotoxicity against patient-matched autologous melanoma cells. Predicting immunodominance may guide the selection of antigens for personalized vaccines and therapies. Another recent study provides an effective pipeline to identify tumor immunogenic epitopes/peptides through improved neoantigen prediction [67].
Translation initiation by m6A-modification
Overview of m6A-modification metabolism in mRNA
Being the most prevalent and highly conserved post-transcriptional modification in eukaryotic mRNA, N6-methyladenosine (m6A) modification plays a crucial role in a wide spectrum of RNA processes, including splicing, nuclear export, RNA degradation and stability, subcellular localization, and translation [68,69,70,71,72,73]. Disruptions in m6A regulation are closely associated with the initiation and progression of cancer by modulating oncogenic signaling pathways, such as Wnt/β-catenin, MAPK, JAK/STAT, PI3K/Akt, and p53 [74], or remodeling the tumor immune microenvironment [75]. Here, our focus will be on exploring the molecular mechanisms of non-canonical translation driven by m6A-modification in cancer progression (Fig. 3). Other types of regulations mediated by m6A-modification in tumorigenesis, development, differentiation, and other diseases have been reviewed elsewhere [68, 76, 77].
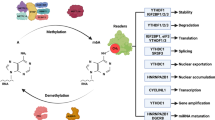
m6A-modification can be employed for translation independently of the cap. A Overview of the control of m6A-modification in the nucleus, involving writers, erasers, and reader, along with their respective inhibitors. B Catalog of cytosolic m6A readers and their inhibitors. C YTHDF1 increases the translation efficiency of m6A-modified mRNAs by interacting with eIF3a/b. YTHDF3 collaborates with YTHDF1 and eIF3a to amplify protein synthesis. D m6A-modification promotes translation by binding with eIF3 in a cap-independent manner. E METTL3 associates with eIF3h and binds to mRNA sites near the translation stop codon, facilitating translation even in the absence of a poly(A)-tail. The necessity for m6A-modification is not fully understood. F YTHDF3 facilitates the binding of eIF3a to the 5′-UTR of mRNAs containing m6A residues through a cap-independent translation mechanism.
The m6A-modification is a reversible and dynamic process, mediated by three categories of proteins: methyltransferases called writers, demethylases called erasers, and binding proteins called readers (Fig. 3A). Writers, exemplified by methyltransferases METTL3 and METTL16, transfer a methyl group from S-adenosylmethionine (SAM) to the N6 position of adenosine residues. Conversely, erasers, such as fat mass and obesity-associated gene (FTO) and α-ketoglutarate-dependent dioxygenase alkB homolog 5 (ALKBH5), remove the methyl group. The m6A-modifications are recognized by readers, which include the YTH domain family of proteins and IGF2BP proteins. After the readers bind m6A-modified mRNA, the mRNA is subsequently directed towards specific RNA processes, such as RNA decay or translation. Thus, the ultimate trajectory of m6A-modified mRNA is predominantly determined by the readers.
In humans, the YTH domain family comprises five members (Fig. 3B): YTHDC1, YTHDC2, YTHDF1, YTHDF2, and YTHDF3. YTHDC1, predominantly located within the nucleus, substantially influences RNA processing. It regulates RNA splicing, ensuring the precise arrangement of exons and introns, and facilitates the export of mature mRNA molecules from the nucleus to the cytoplasm [73, 78]. Conversely, the remaining four YTH proteins primarily reside in the cytoplasm, collectively playing crucial roles in post-transcriptional regulation. Among them, YTHDC2, functioning as an RNA helicase, promotes RNA translation and decay through its helicase activity by unwinding RNA structures for regulatory processes [79,80,81,82].
YTHDF1 enhances translation efficiency by interacting with eIF3A/B and increasing ribosomal engagement (Fig. 3C) [83, 84]. In contrast, YTHDF2 induces degradation by recruiting the CCR4-NOT deadenylase complex [85, 86]. YTHDF3 collaborates with YTHDF1 [87, 88] and eIF3a [88] to boost protein synthesis (Fig. 3C) and influences the decay of methylated mRNA mediated by YTHDF2 [88]. Notably, YTHDF2 and YTHDF3 regulate mRNA localization in neuron in a m6A-depnedent manner [69]. As a whole, the YTH domain family finely regulates post-transcriptional processes, profoundly impacting gene expression through translation, decay, and localization, ultimately shaping cellular function. The distinct roles and precise localizations of these proteins underscore the meticulous nature of the regulatory mechanisms governing m6A-modified mRNA.
Typically, YTH domain family proteins were primarily recognized for their role in modulating canonical translation processes dependent on the cap and poly(A)-tail [79, 83, 87,88,89,90,91,92]. Intriguingly, YTHDF2, conventionally associated with mRNA degradation [85], emerged as the first member of the YTH domain family to be identified as a facilitator of non-canonical translation, particularly in cap-independent translation initiation during heat shock stress [93]. This discovery has fundamentally altered our understanding of its capabilities. Under heat shock stress, YTHDF2 translocates from its usual cytosolic location to the nucleus. Within the nucleus, YTHDF2 competes directly with FTO to protect the 5′-UTR of stress-induced transcripts from demethylation [93]. This protective mechanism enables mRNAs containing m6A in the 5′-UTR, safeguarded by YTHDF2, to be exported and subsequently translated in a cap-independent manner. Subsequent research identified ABCF1 as a pivotal player in m6A-mediated translation, under both heat shock stress and physiological conditions, operating in an eIF4F-independent manner [94].
Meyer et al. also demonstrated that a single m6A residue within the 5′-UTR can enhance translation independently of the cap or the cap-binding protein eIF4E, revealing an additional function of eIF3 as a reader (Fig. 3D) [95]. Moreover, FTO levels negatively regulate translation during stress conditions, such as heat shock, by demethylating m6A in the 5′-UTR [93, 95]. These studies open avenues for exploring the broader role of 5′-UTR m6A-modification in cap-independent translation during stress and highlight the importance of investigating the detailed connection between human diseases and cap-independent translation facilitated by 5′-UTR m6A.
Translation of m6A-modified mRNAs in cancers
While numerous studies have underscored the direct influence of altered m6A RNA metabolism on tumor progression and the acquisition of stem-like characteristics in cancer cells [74, 75, 77, 96, 97], most of these investigations lack an in-depth elucidation of the precise molecular mechanisms governing translational processes via m6A-modification in mRNAs. Consequently, distinguishing between canonical and non-canonical translation driven by m6A-modification proves to be a challenging task in the majority of studies, although there are some examples where distinct mechanisms are discernible (Table 1).
Lin et al. and Choe et al. made the significant discovery that METTL3, a key writer enzyme, interacts with the translation initiation machinery, particularly eIF3h. This interaction leads to the enhanced translation of a specific subset of mRNAs, facilitated by METTL3 binding to mRNA sites near the translation stop codon (Fig. 3E) [98, 99]. The mechanism underlying this enhancement involves the promotion of mRNA circularization in a cap-dependent manner, even in the absence of a poly(A)-tail [98]. Interestingly, it doesn’t necessitate the m6A catalytic activity of METTL3. Rather, the N-terminus region (aa 1-200) of METTL3 alone is sufficient for this augmented mRNA translation [98, 99].
While METTL3 is associated with the translation enhancement of a substantial portion of mRNAs, and m6A peaks are predominantly found in proximity to stop codons, METTL3 binding only occurs at about 22% of methylated GGAC sites. Moreover, other readers like YTHDF1 and YTHDF2 are not implicated in this translation mechanism. This suggests the possible existence of additional components that assist in tethering METTL3 to specific target sites [99]. As a result, whether m6A-modification near stop codons is an absolute requirement is still subject to further clarification.
In terms of its impact on cancer biology, METTL3 exerts control over the translation of numerous target mRNAs involved in tumor progression and apoptosis. Elevated METTL3 expression has been observed in lung and colon cancers. Depleting METTL3 leads to a significant decrease in the growth, invasion potential, and 3D soft agar colony formation of cancer cells in vitro, as well as a reduction in tumor size in mouse xenografts originating from A549 lung cancer cells. Conversely, elevating METTLL3 levels in human and mouse fibroblast cell lines effectively stimulates cell invasion, a crucial characteristic for tumor advancement. Notably, suppressing METTL3-mediated translation through eIF3h knockdown substantially curtails cell invasion. Furthermore, the capacity of METTL3 to enhance cell invasion and colony formation is nullified by a mutation at A155P, which disrupts the interaction between METTL3 and eIF3h, thereby impeding METTL3-mediated translation [98]. These findings highlight the significant role of METTL3 in advancing tumorigenesis via an mRNA looping mechanism that operates independently of a poly(A)-tail.
A recent study revealed a significant upregulation of YTHDF3 expression in brain metastases originating from breast cancer, as opposed to metastases in other tissues [100]. This heightened expression correlated with a negative impact on survival rates in both breast cancer patients and mouse models, indicating a promotion of brain metastasis. YTHDF3 was found to enhance the translation of its own mRNA, along with m6A-transcripts of pivotal genes associated with brain metastasis, including ST6GALNAC5, GJA1, EGFR, VEGFA, and SRC. Notably, YTHDF3 facilitated the binding of eIF3a to the 5′-UTR of mRNAs containing m6A residues, independent of eIF4E, implying a cap-independent translation mechanism (Fig. 3F).
Benavides-Serrato et al. demonstrated that m6A-modification enhances the translation of cyclin D1 and c-Myc in GBM cancer cells [101]. Intriguingly, this mechanism functions through m6-modification within the IRES elements of their 5′-UTRs, which is recognized by YTHDF3. The heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) interacts with both YTHDF3 and IRES, leading to enhanced translation and increased resistance of GBM to mTOR inhibitor.
Prospects for cancer therapy targeting m6A-modification
Numerous investigations have underscored the role of m6A-modification and its regulatory proteins across diverse human cancers, influencing critical aspects like tumorigenesis, metastasis, resistance, and immunoregulation [75, 102, 103]. Genetic manipulation of writers, erasers, or readers has demonstrated promising results in diverse cancer models [103,104,105]. Nevertheless, the availability of pharmacological inhibitors remains limited, targeting only a select group of regulators (Fig. 3).
Treatment of STM2457, an inhibitor targeting the methyltransferase activity of METTL3, results in a specific and significant reduction in stem cell populations within acute myeloid leukemia (AML) [106]. This intervention also resulted in a prolonged lifespan for mice without causing discernible toxicity to normal hematopoiesis.
Several inhibitors targeting FTO have been developed, including small-molecule compounds like FB23 and FB23-2. These inhibitors effectively suppress the proliferation of AML cells. Notably, FB23-2 effectively suppresses the growth of human AML cell lines in vitro and inhibits patient-derived primary xenotransplantation (PDX) AML models in vivo [107]. Xiao et al. demonstrated that an antigen-capturing nanoplatform, which concurrently delivers tumor-associated antigens and the FTO inhibitor (FB23-2) into tumor-infiltrating dendritic cells, significantly enhances tumor-specific immune responses both in vivo and in vitro [108].
The recently disclosed FTO inhibitors, CS1 and CS2, exhibit impressive effectiveness in suppressing the proliferation of AML cells in vitro and in PDX models in vivo by targeting FTO’s demethylation activity [109]. Notably, compounds CS1 and CS2 demonstrate anti-tumor potential over ten times higher than that of FB23-2 (>1 µM) in AML cells, with IC50 values in the nanomolar range.
Dac51, an FTO inhibitor, enhances immunotherapy efficacy by countering FTO-mediated immune evasion [110]. It reprograms RNA epitranscriptome, a potential immunotherapy strategy. Dac51 also prevents tumor cells from evading CD8+ T cell surveillance by regulating glycolytic metabolism through FTO-mediated m6A modifications.
Meclofenamic acid (MA), identified as an FTO-specific inhibitor, exhibits selectivity over ALKBH5 [111]. In a study, MA2-treated mice showed notably reduced tumor size and prolonged survival compared to the control group, underscoring the effectiveness of FTO inhibition in glioblastoma stem cell (GSC) models. This highlights the potential of FTO inhibitors in impeding the progression of GSC-initiated tumors [112].
In contrast to FTO inhibitors, reports on ALKBH5 inhibitors are limited. Nevertheless, Compound 3 and 6, identified through high-throughput virtual screening, demonstrated the ability to inhibit cell proliferation in leukemia cell lines [113]. Similarly, inhibition of ALKBH5 using Ena15 or Ena21, identified through separate screen of small-molecule compounds, resulted in inhibited cell proliferation in glioblastoma multiforme-derived cell lines [114].
Recent advancements in cancer research have unveiled novel YTHDF inhibitors with significant therapeutic potential. Tegaserod, identified through targeted screening of FDA-approved drugs, disrupts the direct binding of YTHDF1 with m6A-modified mRNAs, effectively impeding YTHDF1-mediated translation of cyclin E2 [97]. This compound also demonstrates reduced viability of patient-derived AML cells in vitro and extended survival in PDX models.
DC-Y13-27, a potent YTHDF2 inhibitor, enhances tumor response to ionizing radiation (IR) in myeloid cells [115]. By overcoming myeloid-derived suppressor cells (MDSC)-induced immunosuppression, it improves the effectiveness of combined IR and/or anti-PD-L1 therapy, highlighting YTHDF2 as a promising target for radiotherapy and radioimmunotherapy combinations.
Ebselen, a broad-spectrum YTH domain inhibitor, disrupts the interaction between the YTHDF m6A domain and its mRNA targets [116]. While its anticancer activity remains untested, it exhibits potential for treating specific cancers like AML, gastric carcinoma, and HCC.
While the potential for cancer treatment through targeting METTL3 and METTL16 has been extensively demonstrated, it is important to note that these genes are essential for cell survival [105, 117]. Consequently, pharmacological inhibition of METTL3, whether by STM2457 or other potential inhibitors, may lead to significant toxicity. Nevertheless, careful adjustment of inhibition levels or drug concentrations could help mitigate this concern.
Interestingly, both METTL3 and METTL16 have been found to participate in translation independently of m6A-modification, contributing to tumorigenesis [98, 105], despite their primary roles as m6A writers and their predominant action via m6A. Given the increasing evidence emphasizing the significance of translation control by METTL3 and METTL16, especially in cancer contexts, developing drugs that selectively target their translation-related activity while preserving their methylation function could offer a promising therapeutic avenue. Additionally, considering the non-canonical translation mechanisms of m6A mRNA through interactions with METTL3 or YTHDF1/3 with eIF3, the development of drugs targeting this interaction holds potential for anticancer treatment. Despite the challenges of harnessing m6A-modification for cancer therapy, it represents a promising new approach with the potential to revolutionize cancer care in the future.
Translation of circRNAs
Circular RNAs (circRNAs), ncRNAs generated through back-splicing, were initially discovered in viruses in 1976 and have since been identified in a variety of organisms, tissues, and cell types [118,119,120]. While the specific functions of many circRNAs still require further study, they are commonly regarded as sponges for microRNAs and proteins [118, 119, 121]. Unlike typical mRNA, circRNAs lack both a cap and a poly(A)-tail, classifying them as ncRNA molecules. The potential for circRNA translation is still under discussion and requires thorough exploration [122, 123], yet mounting evidence indicates that certain circRNAs contain ORFs and can encode peptides/proteins via cap-independent translation mechanisms [14, 118, 120]. Historically, proteins encoded by ORFs shorter than 100-aa were excluded from protein databases, but circRNAs often contain such short ORFs. Recent advancements in bioinformatics, translational research, and proteomics have revealed numerous peptides/proteins encoded by circRNAs [124,125,126]. The involvement of circRNA-produced peptides/proteins has been noted in several disease contexts, including Alzheimer’s disease [127, 128], cardiovascular diseases [129, 130], pulmonary fibrosis [131], and various types of cancer [120].
In this section, we will delve into the molecular mechanisms, key recent discoveries, and therapeutic implications of circRNA-driven translation in cancer progression. The biogenesis, detection, biomarkers, and other regulatory functions of circRNAs, including their role as microRNA sponges in cancer, are covered in other reviews [119, 121].
Mechanisms of circRNA translation
In contrast to traditional translation mechanisms, uncapped circRNAs initiate translation through two non-canonical translation mechanisms: IRES [132] and MIRES (m6A-induced ribosome engagement site [95]) (Fig. 4) [14, 120].
The translation of circRNAs occurs independently of a cap and poly(A)-tail. A IRES-mediated non-canonical translation is a prevalent mechanism in circRNA, circumventing the need for a cap and several eIFs. B YTHDF2 or YTHDF3 recognizes m6A-modified residues, recruiting initiation factors such as eIF4G2 and 43S PIC, which then initiates translation. C In cases where a circRNA lacks a stop codon and possesses a nucleotide count that is a multiple of three, it allows for limitless translation, potentially leading to the production of polymeric structural proteins through infinite rolling-circle translation (RCT). D EJC deposited during back-splicing promotes the initiation of circRNA translation through the interaction between eIF4A3 and eIF3g. E The diagram outlines potential therapeutic strategies for anticancer interventions that utilize circRNA translation.
IRES, a well-studied cis-element in cap-independent translation, was initially identified in the poliovirus [133, 134] and later discovered in both viral (vIRES) and cellular (cIRES) genes [1, 2, 135, 136]. Typically located in the 5′-UTR and rarely in CDS regions [137], IRES elements enable mRNAs to directly recruit the 40S ribosomal subunit for translation initiation. IRES translation requires only a subset of eIFs and operates independently of the cap [1, 2]. IRES interacts with eIF4G1 (also called eIF4GI or p220)/eIF4G2 (also called DAP5 or p97) [138,139,140,141,142], or the eIF3 complex [142,143,144], leading to the recruitment of 40S subunits and the subsequent formation of 43S PIC to initiate translation (Fig. 4A).
Cap-independent translation enhancers (CITEs) are structural RNA elements with diverse structures that facilitate translation initiation by binding to specific eIFs such as eIF4G1, eIF4G2/DAP5, eIF4G3, eIF4E, and eIF3, as well as 18S rRNA [145,146,147]. CITEs are similar to IRESs in that both mechanisms enable mRNA translation without requiring a cap structure and eIF4E. However, CITEs require a free or exposed 5′ end and employ ribosome scanning to locate the start codon. The first CITE, known as the translation enhancer domain (TED), was identified in satellite Tobacco necrosis virus (sTNV) [148]. While numerous studies have reported cap-independent or IRES-mediated translation in mammalian cells, only a few have provided convincing or direct evidence of CITEs functioning via eIF4G1 or eIF4G2 [138, 149, 150]. Although it is highly plausible that cells under stress, such as cancer cells under hypoxia, may employ cap-independent translation mechanisms like CITEs, there is currently no report directly linking CITE regulation to cancer or other pathological conditions. Additionally, CITEs can be located in both the 5′- and 3′-UTRs of mRNA, with a predominance in the 3′-UTR. However, in mammalian cells, only a few 5′-UTRs, such as those from HIF1A, TP53, and APAF1, have been identified as CITEs, while no 3′-UTR CITEs have been discovered. This discrepancy may be due to the overestimation of IRESs and insufficient experimental validation. Therefore, identifying cellular CITEs and understanding their involvement in physiological processes are important directions for future research, and our review will not include details of CITEs.
Given that circRNAs lack both a cap and a poly(A)-tail, they exclusively undergo cap/poly(A)-independent translation [1, 120]. Therefore, IRESs play a crucial role in translation initiation for circRNAs. Chen et al. discovered over 17,000 native or synthetic sequences functioning as IRESs for circRNAs through a split-eGFP reporter system containing an IRES oligo library. They demonstrated that circRNA translation via IRES involves base-pairing between the IRES and the active regions of the 18S rRNA and a structured RNA element within the IRES [132]. With the increasing recognition of the biological significance of circRNA translation, a recent study introduced DeepCIP, a deep learning approach, to predict IRES elements in circRNAs by combining two deep neural network modules, Sentence-State LSTM (S-LSTM) and Graph Convolutional Networks (GCN) [151]. Although requiring further improvement, this method will enhance the understanding of translation mechanisms of circRNAs.
IRES transacting factors (ITAFs) play important roles in modulating IRES-driven translation by stabilizing IRES structures or inducting necessary conformation changes, thereby promoting the recruitment of ribosomal subunits. While most identified ITAFs are associated with linear mRNA, their roles and mechanisms in IRES-mediated translation have been discussed elsewhere [152]. However, some ITAFs have been identified from circRNA translation. PABPC1/4 and hnRNP U facilitate translation by recognizing IRES-like elements, especially AU-rich sequences, in circRNA [125]. Although IRES-mediated non-canonical translation is a major mechanism in circRNA translation, the comprehensive regulatory mechanisms by IRES and specific ITAFs involved still demand further exploration.
m6A methylation is notably more prevalent in circRNAs than in mRNAs [153, 154], with METTL3 and YTHDC1 positively influencing the biogenesis of circRNAs through m6A-modification [154, 155]. This m6A-modification plays a crucial role in enabling cap-independent translation by acting as a MIRES. Within the 5′-UTR, m6A can interact with YTHDF3 or eIF3, facilitating the recruitment of the 43S PIC independently of the cap, thereby initiating translation (Fig. 3D, F) [95, 100]. Therefore, it is plausible that RNA elements modified with m6A may exhibit similar MIRES-initiated translation activity in circRNA. Supporting this hypothesis, studies have shown that m6A sites in circRNAs can be recognized by YTHDF3, which, upon binding to eIF4G2, recruits the 40S subunit for translation initiation (Fig. 4B) [153, 155]. Remarkably, a single m6A-modification in circRNA is sufficient to initiate translation with the cooperation of eIF4G2 and YTHDF3 [153]. The removal of m6A by the demethylase FTO decreases circRNA translation, whereas co-expression with the m6A methyltransferases METTL3/14 leads to an increase. Furthermore, a reduction in YTHDF3 levels results in decreased circRNA translation [153]. A recent study also discovered that YTHDF2, rather than YTHDF3, facilitates m6A-dependent translation of circMET in GSCs [156]. Moreover, another investigation revealed that circMAP3K4 produces the circMAP3K4-455aa protein through m6A-modification, which is recognized by IGF2BP1, thereby facilitating the progression of HCC [157]. This finding provides a new insight into m6A-mediated translation in circRNA, although the exact function of IGF2BP1 in circRNA translation remains incompletely understood.
Rolling circle translation (RCT) represents a distinctive translation process for circular RNAs that do not contain a stop codon, enabling continuous translation due to an uninterrupted ORF (Fig. 4C). This mode of translation, reminiscent of a polymerase reaction, occurs when a circRNA is devoid of a stop codon and features a nucleotide sequence that is a multiple of three, creating an infinite ORF (iORF). Theoretically, this allows for endless translation, ultimately leading to the production of polymeric structural proteins as demonstrated in Escherichia coli and mammalian cells in live-cell or cell-free systems [158,159,160,161]. Similarly, a recent study showed that circEGFR employs infinite RCT to encode a rolling-translated EGFR (rtEGFR), resulting in the formation of a novel polymeric protein complex. The programmed -1 ribosomal frameshifting (-1PRF) mechanism, which generates an out-of-frame stop codon, was found to facilitate translation termination [162]. Fan et al. reported that approximately 67% of endogenous circRNAs have the capacity to encode proteins exceeding 20-aa, overlapping with their corresponding genes. An additional 27% of circRNAs also exhibit translatability, leaving only 7% of circRNAs without any identifiable ORF. Intriguingly, analysis from public datasets of mass spectrometry suggests that around 50% of translatable circRNAs can generate protein concatemers through the RCT mechanism [125]. In addition, a recent study predicted that circASPH splice variants lacking a stop codon may undergo infinite RCT, producing large proteins, though experimental validation is required [163]. This non-classical RCT translation mechanism introduces a potential third pathway in circRNA, distinct from IRES- and m6A-mediated translations. However, the identification of RCT products of endogenous circRNAs is limited, and further exploration is needed to unravel their biological functions and underlying mechanisms.
Additionally, two recent studies have demonstrated that the exon-junction complex (EJC), which is deposited during back-splicing, facilitates ribosome recruitment to circRNA through the interaction between eIF4A3 and eIF3g, thereby enhancing translation initiation (Fig. 4D) [164, 165]. However, further research is needed to thoroughly address the many remaining questions. How does the EJC selectively activate a subset of circRNAs, given that almost all circRNAs are produced by back-splicing? Does the EJC work in conjunction with IRES or m6A-driven translation mechanisms? To what extent does the EJC contribute to circRNA translation? Does the EJC remain attached following the initial round of translation? Importantly, do circRNA translations driven by the EJC have significant roles in cellular functions or disease progression?
Translation of circRNAs in cancer
In this section, we will highlight significant discoveries in circRNA translation, as a growing body of research emphasizes its clinical importance in many cancers (Table 1).
GBM, a grade IV astrocytoma, is an aggressive brain cancer with a survival rate of less than 10% beyond five years after diagnosis. PINT87aa, encoded by circRNA LINC-PINT, restrains GBM cell proliferation in vitro and in vivo [166]. It directly interacts with the polymerase-associated factor complex (PAF1c) in the nucleus, impeding the transcriptional elongation of multiple oncogenes, including c-Myc. Both PINT87aa and LINC-PINT are reduced in GBM compared to normal tissue levels, suggesting the involvement of functional peptides encoded by circRNA in GBM tumorigenesis.
The ORF in circ-SHPRH produces SHPRH-146aa, a functional 17 kDa protein via IRES. Both circ-SHPRH and SHPRH-146aa exhibit reduced expression in GBM [167]. SHPRH-146aa functions as a GBM tumor suppressor by protecting SHPRH, a known tumor suppressor, from ubiquitination. This protection leads to the inhibition of cell proliferation and tumorigenicity in vitro and in vivo, as seen in experiments with U251 and U373 GBM cell lines. Both SHPRH-146aa and SHPRH expression levels are positively correlated with a better prognosis for patient with GBM.
Circ-FBXW7 carries an IRES-driven transjunctional ORF that encodes FBXW7-185aa [168]. Elevated levels of FBXW7-185aa inhibit GBM cell proliferation, while decreased levels promote malignancy in vitro and in vivo. FBXW7-185aa reduces c-Myc stability by counteracting the activity of the de-ubiquitinating enzyme USP28, which stabilizes c-Myc through de-ubiquitination. In GBM clinical samples, both circ-FBXW7 and FBXW7-185aa levels are reduced. Importantly, circ-FBXW7 shows a positive correlation with overall patient survival.
Another circRNA, circ-AKT3, produces AKT3-174aa, which competes with AKT by interacting with PDK1. This competition results in decreased Akt-Thr308 phosphorylation and PI3K/AKT signaling [169]. Consequently, it suppresses GBM cell proliferation, radiation resistance, and tumorigenicity. On the contrary, when circ-AKT3 is downregulated, it promotes cell malignancy.
CRC stands as the third most prevalent cancer and the second highest contributor to cancer-related fatalities worldwide. One notable circRNA, circPLCE1, produces a 411-aa protein circPLCE1-411, driving the dissociation of RPS3 from the HSP90α/RPS3 complex [170]. This triggers RPS3 degradation, a pivotal regulator of NF-κB. As a result, circPLCE1-411 inhibits NF-κB nuclear translocation in CRC cells, suppressing both proliferation and metastasis. These findings were confirmed across multiple CRC models.
Another circRNA, circFNDC3B, exhibits reduced expression in both CRC cell lines and patient tissues [171]. This circRNA produces circFNDC3B-218aa, functioning as a tumor suppressor by inhibiting CRC proliferation, invasion, and migration. The tumor-inhibitory effect of circFNDC3B-218aa is mediated through the suppression of Snail expression. This leads to the upregulation of FBP1, a recognized tumor suppressor, further enhancing its tumor-suppressive impact in CRC.
Similarly, the expression of circMAPK14 is significantly decreased in CRC cells and tissues. It is primarily localized in the cytoplasm and encodes a 175aa peptide, circMAPK14-175aa [172]. This peptide acts as a tumor suppressor by reducing the nuclear translocation of MAPK14 through competitive binding to MKK6, thereby facilitating the ubiquitin-mediated degradation of FOXC1. Additionally, there is a positive feedback loop in CRC, where reduced circMAPK14-175aa expression leads to elevated FOXC1 expression. This, in turn, suppresses U2AF2 transcription, leading to decreased circMAPK14 biogenesis.
Lung cancer has the highest death rate among all cancers globally. In the intricate landscape of lung adenocarcinoma (LUAD), the alternative Wnt pathway is a pivotal player by sustaining stem cell renewal and fostering resistance. Notably, the diminished expression of circ-FBXW7 is implicated in resistance to Osimertinib, a third-generation EGFR inhibitor and targeted therapy for lung cancers, including LUAD [173]. Circ-FBXW7 inhibits stem cell renewal, enhancing the response to Osimertinib. It generates circ-FBXW7‑185AA, also known as FBXW7-185aa [170], in an m6A and YTHDF3-dependent manner. This peptide interacts with β‑catenin, suppressing Wnt signaling and leading to increased Let-7d miRNA expression. Let-7d, in turn, reduces YTHDF3 levels, establishing a negative feedback loop. This study reveals the mechanism behind LUAD stem cell resistance to Osimertinib, providing insights into potential therapeutic strategies.
In gastric cancer, or stomach cancer, circDIDO1 plays a crucial role. Downregulated in gastric cancer tissues, circDIDO1 hinders gastric cancer cell proliferation, migration, and invasion [174]. Its overexpression leads to reduced tumor growth and metastasis. CircDIDO1 contains IRES, ORF, and m6A-modification, generating a protein called DIDO1-529aa. This protein interacts with PARP1, inhibiting its activity, and targets PRDX2 for degradation, deactivating downstream pathways. This discovery underscores the tumor-suppressive role of DIDO1-529aa in gastric cancer.
HCC or liver cancer is the third most common cause of cancer-related deaths worldwide. circARHGAP35, derived from ARHGAP35 mRNA through back-splicing facilitated by HNRNPL, possesses a 3867 nucleotide ORF initiated by m6A-modification. The resulting circARHGAP35 protein, a truncated form of ARHGAP35, activates oncogenes by binding to TFII-I via FF domains within the nucleus, correlating with an unfavorable prognosis in HCC patients [175].
CircSTX6, along with its 144aa peptide circSTX6-144aa, is highly expressed in HCC tissues and serves as an independent risk factor for patient survival [176]. METTL14 regulates circSTX6 expression through an m6A-dependent mechanism. Functionally, circSTX6 promotes HCC proliferation, tumorigenicity, and metastasis. Mechanistically, it acts as a sponge for HNRNPD, promoting HNRNPD-mediated ATF3 mRNA decay. Additionally, circSTX6-144aa independently drives HCC progression. This highlights their potential as biomarkers and therapeutic targets in HCC.
A tumor-suppressive circRNA, circZKSCAN1, is significantly underexpressed in HCC samples and encodes a 206-aa protein circZKSaa, which is predominantly located in the cytoplasm and can be secreted [177]. circZKSaa effectively inhibits the growth of HCC cells in vitro and in vivo by promoting the degradation of mTOR through interactions with both mTOR and FBXW7.
In bladder cancer, circGprc5a is upregulated in tumors and cancer stem cells (CSCs), with its overexpression boosting the CSC ratio via the circGprc5a-peptide encoded by circGprc5a [178]. The study reveals a critical interaction between circGprc5a-peptide and Gprc5a, a protein crucial for bladder CSC maintenance. Conversely, mutant circGprc5a or overexpression of circGprc5a in the absence of Gprc5a does not affect CSC ratios. This underscores the therapeutic potential for bladder cancer by targeting circGprc5a-peptide, Gprc5a, or their interaction.
In breast cancer, circSEMA4B was notably decreased in tumor tissues and cell lines and acts as a tumor suppressor through two distinct mechanisms [179]. First, circSEMA4B sponges miR-330-3p, resulting in the upregulation of the tumor suppressor PDCD4 and subsequent inhibition of AKT signaling. Second, SEMA4B-211aa, encoded by SEMA4B, indirectly interacts with free p85, a regulatory subunit of PI3K, outcompeting the catalytic subunit p110. This interaction suppresses PI3K activity and the downstream AKT signaling. This study sheds light on the modulation of the PI3K/AKT signaling pathway as a strategy against breast cancer progression.
Potential in cancer therapy targeting circRNA translation
The translation of circRNAs is crucial in cancer progression, yet there are currently no specific inhibitors or modulators to regulate circRNAs processes like IRES or MIRES. Inhibiting the translational synthesis of tumor-promoting peptides/proteins encoded by circRNAs could effectively prevent cancer (Fig. 4E). Small-molecule inhibitors have shown the capability to hinder the translation of IRES-containing transcripts such as IGF1R and c-Myc, without impeding global cap-dependent translation [180, 181]. However, the vast diversity of IRES sequences in circRNAs, along with vIRES and cIRES, may render it challenging to develop small molecules targeting each specific IRES. Alternatively, antisense oligonucleotides (ASOs) or peptide nucleic acids (PNAs) can precisely target individual IRES sequences [181, 182]. Targeting the interaction between IRESs and ITAFs holds promise as a potential avenue for developing specific small molecule inhibitors such as riluzole [181, 183]. Nevertheless, these IRES inhibitors have not been tested for their ability to inhibit circRNA translation. Additionally, several drugs targeting m6A-modification are available, as described in section “Prospects for cancer therapy targeting m6A-modification”. It may be worthwhile to investigate whether pharmacological inhibition of IRES or m6A-modification could effectively prevent circRNA translation, and thereby suppress tumor progression.
On the other hand, enhancing the expression of tumor-suppressing peptides/proteins or circRNAs through increased circRNA expression is a conceivable means to more effectively combat cancers. Lipid nanoparticles (LNPs), enclosed spherical lipid vesicles, are widely used in the preclinical or clinical setting as carriers for drugs, including circRNA [184,185,186]. For instance, circFoxo3 was delivered through plasmids conjugated with gold nanoparticles to induce tumor apoptosis [187]. Additionally, overexpression of circ-1073 inhibited breast cancer cell proliferation and induced apoptosis. The growth of xenograft tumors was suppressed by intratumoral injection of nanoparticles containing circ-1073 [188].
Targeting critical signaling pathways influenced by oncogenic peptides or proteins produced by circRNAs offers substantial promise for cancer therapy. Here, we highlight examples of circRNA-encoded peptides/proteins that act as oncogenic molecules and explore their therapeutic implications. Furthermore, the translation products from circRNAs may serve as neoantigens for cancer immunotherapy, as detailed in section “Prospects for cancer therapy involving non-canonical ORFs”. For more information on neoantigens, please refer to that section.
Dysregulated activation of the Hedgehog pathway has been associated with various cancers, including medulloblastoma, gastric cancer, HCC, pancreatic cancer, and others. Circ-SMO generates SMO-193aa, essential for initiating Hedgehog signaling in GBM [189]. SMO-193aa promotes GBM progression. Diminished Hedgehog signaling in GSCs hampers self-renewal, in vitro proliferation, and in vivo tumorigenicity. Targeting circ-SMO and/or SMO-193aa could be a promising therapeutic approach for GBM treatment.
EGFR and other members of the ErbB/HER family are closely linked proteins essential for the regulation of cell growth and division. Alterations such as mutations or increased expression of these receptors can drive cancer advancement. Cancer types associated with EGFR/HER modifications include non-small cell lung cancer, GBM, breast cancer, and various others [190]. Circ-E-Cad RNA undergoes successive rounds of ORF translation, facilitated by the absence of a stop codon in the initial round read. This process leads to the generation of a novel secreted E-cadherin protein variant, C-E-Cad [191]. C-E-Cad binds to the EGFR CR2 domain via a 14-aa carboxyl terminal, activating EGFR independently of EGF and thus contributing to the maintenance of the tumorigenicity of GSCs. C-E-Cad is overexpressed in GBM, further enhancing the tumorigenicity of GSCs.
Another circRNA, circ-EGFR, utilizes an iORF to generate rtEGFR through infinite RCT [162]. rtEGFR interacts with EGFR, maintaining its membrane localization and reducing endocytosis and degradation. Depletion of rtEGFR in brain tumor initiation cells reduces tumorigenicity and enhances anti-GBM effects.
Circ-HER2, found in 30% of TNBC cases, produces a 103-aa protein, HER2-103 [192]. TNBC patients with circ-HER2/HER2-103-positive status often exhibit poorer prognoses. Suppressing circ-HER2 hinders TNBC cell behaviors, underscoring its pivotal role in tumorigenesis. Mechanistically, HER2-103 drives EGFR/HER3 dimerization, sustaining AKT activation and promoting malignancy. HER2-103 shares sequences with the HER2 CR1 domain, which is a target of pertuzumab. Pertuzumab significantly reduces in vivo tumorigenicity of circ-HER2/HER2-103-positive TNBC cells. These discoveries present a potential approach for cancer treatment by targeting EGFR/HER.
The Hippo-YAP pathway, which governs cell growth, proliferation, and organ proportions, is implicated in various types of cancer, including HCC, breast cancer, CRC, and others. In colon cancer, circPPP1R12A is specifically upregulated. It encodes the functional peptide circPPP1R12A-73aa, which enhances colon cancer growth and metastasis by activating the Hippo-YAP signaling pathway [193]. Patients with elevated levels of circPPP1R12A exhibit markedly reduced overall survival. Importantly, the YAP-specific inhibitor, Peptide 17, substantially attenuates the cancer-promoting impact of circPPP1R12A-73aa on colon cancer cells, suggesting that Hippo-YAP inhibition could serve as a therapeutic strategy for this type of cancer.
The Wnt/β-catenin pathway is pivotal in regulating cell growth and differentiation. Abnormal activation of this pathway is associated with various cancers, including colorectal, breast, liver, and lung cancers. In liver cancer, circβ-catenin is expressed at high levels, and silencing circβ-catenin significantly suppresses malignant characteristics both in vitro and in vivo [194]. A novel 370aa β-catenin variant, β-catenin-370aa, encoded by circβ-catenin, stabilizes full-length β-catenin by counteracting its phosphorylation and degradation by GSK3β. As a result, the Wnt pathway is activated, promoting tumor growth in liver cancer.
In TNBC, circ-EIF6 is correlated with poor prognosis due to its enhancement of cancer cell growth and metastasis [195]. An IRES within circ-EIF6 facilitates the expression of EIF6-224aa, which interacts with MYH9, a known oncogene. This interaction stabilizes MYH9 and activates the Wnt pathway, contributing to the oncogenic effects of circ-EIF6 in TNBC.
Additionally, circAXIN1, highly expressed in gastric cancer tissues and cell lines, encodes a 295aa protein, AXIN1-295aa [196]. The targeted silencing of circAXIN1 using siRNA significantly diminishes cancer-related traits such as cell growth, migration, invasion, and colony formation. In stark contrast, exogenous expression of AXIN1-295aa accelerates cancer advancement. The oncogenic role of AXIN1-295aa stems from its interaction with APC, a component crucial for the degradation of β-catenin. This interaction leads to a decrease in the binding between AXIN1 and APC, consequently elevating β-catenin activity, which further contributes to the progression of gastric cancer. These findings underscore the potential of targeting the Wnt/β-catenin signaling pathway as a treatment strategy for this type of cancer.
MET, a key receptor tyrosine kinase for GBM, is activated by the hepatocyte growth factor (HGF). A recent study found that circMET produces a MET variant, MET404, via m6A-modification, driven specifically by YTHDF2 instead of YTHDF1/3, which are known m6A readers for m6A-mediated translation [156]. MET404 is often found to be overexpressed in GBM cases. It acts as a secreted protein that binds to the MET β subunit, leading to constitutive activation of the MET receptor in GBM, a significant factor in GBM tumorigenesis and associated with a poor prognosis. Targeting MET404 with a neutralizing antibody, or using the FDA-approved MET inhibitor, onartuzumab, either alone or in combination, results in decreased tumor growth and extended survival in vivo. This suggests a potential treatment approach for GBM.
A comprehensive understanding of complex signaling networks and their dysregulation in various cancers is essential for developing safer and more effective therapeutic approaches. As research advances, the potential to harness circRNAs and their translated products for targeted cancer interventions is continually expanding, paving the way for improved cancer treatments.
Conclusion and future directions
In recent years, a surge of research has illuminated various non-canonical translation mechanisms, diverging from the classical paradigm dependent on a cap, AUG start codon, stop codon, and poly(A)-tail. Cancer cells employ various non-canonical translation mechanisms to ensure survival, maintain stemness, and adapt to different environments, including the development of drug resistance.
This review highlights recent progress in non-canonical translation mechanisms, underscoring their emerging therapeutic potential in oncology. The exploration of these mechanisms, including non-canonical ORFs, m6A-modification, and circRNAs, holds the promise of unveiling uncharted territories in the human proteome. Novel isoforms and proteins arising from these unconventional processes may have dual roles in tumorigenesis. Targeting the translation machinery or modulating signaling pathways governed by tumor-promoting peptides/proteins shows promise for cancer therapy. For peptides/proteins that suppress tumors, activating the critical downstream pathway or delivering the peptide or circRNA could be a viable target for drug development. However, our comprehension of these mechanisms in cancer is progressing, necessitating further in-depth research.
Moreover, an imperative task lies in delineating the precise roles and implicated signaling pathways of these novel peptides/proteins across diverse biological contexts within the cancer milieu. Despite these challenges, conceptualizing and implementing a diverse array of strategies to impede non-canonical translation pathways offers profound potential as a therapeutic avenue in the expansive landscape of cancer treatment.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Brito Querido J, Díaz-López I, Ramakrishnan V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat Rev Mol Cell Biol. 2024;25:168–86.
Leppek K, Das R, Barna M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat Rev Mol Cell Biol. 2018;19:158–74.
Passmore LA, Coller J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat Rev Mol Cell Biol. 2022;23:93–106.
Lacerda R, Menezes J, Romão L. More than just scanning:the importance of cap-independent mRNA translation initiation for cellular stress response and cancer. Cell Mol Life Sci. 2017;74:1659–80.
Smith RCL, Kanellos G, Vlahov N, Alexandrou C, Willis AE, Knight JRP, et al. Translation initiation in cancer at a glance. J Cell Sci. 2021;134:jcs248476.
Jin YN, Schlueter PJ, Jurisch-Yaksi N, Lam P-Y, Jin S, Hwang WY, et al. Noncanonical translation via deadenylated 3′ UTRs maintains primordial germ cells. Nat Chem Biol. 2018;14:844–52.
Jin YN, Peterson RT. Chemical genetics: manipulating the germlines with small molecules. In: Dosch R, editor. Germline development in the zebrafish: methods and protocols. New York, NY: Springer US; 2021. p. 61–73.
Cao X, Slavoff SA. Non-AUG start codons: expanding and regulating the small and alternative ORFeome. Exp Cell Res. 2020;391:111973.
Kearse MG, Wilusz JE. Non-AUG translation: a new start for protein synthesis in eukaryotes. Genes Dev. 2017;31:1717–31.
Andreev DE, Loughran G, Fedorova AD, Mikhaylova MS, Shatsky IN, Baranov PV. Non-AUG translation initiation in mammals. Genome Biol. 2022;23:111.
Malik I, Kelley CP, Wang ET, Todd PK. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat Rev Mol Cell Biol. 2021;22:589–607.
Zhou Z-D, Jankovic J, Ashizawa T, Tan E-K. Neurodegenerative diseases associated with non-coding CGG tandem repeat expansions. Nat Rev Neurol. 2022;18:145–57.
Cui L, Ma R, Cai J, Guo C, Chen Z, Yao L, et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduct Target Ther. 2022;7:1–26.
Wang Y, Wu C, Du Y, Li Z, Li M, Hou P, et al. Expanding uncapped translation and emerging function of circular RNA in carcinomas and noncarcinomas. Mol Cancer. 2022;21:13.
Wright BW, Yi Z, Weissman JS, Chen J. The dark proteome:translation from noncanonical open reading frames. Trends Cell Biol. 2022;32:243–58.
Leong AZ-X, Lee PY, Mohtar MA, Syafruddin SE, Pung Y-F, Low TY. Short open reading frames (sORFs) and microproteins:an update on their identification and validation measures. J Biomed Sci. 2022;29:19.
Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng X, et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22.
Dever TE, Ivanov IP, Sachs MS. Conserved upstream open reading frame nascent peptides that control translation. Annu Rev Genet. 2020;54:237–64.
Jürgens L, Wethmar K. The emerging role of uORF-encoded uPeptides and HLA uLigands in cellular and tumor biology. Cancers. 2022;14:6031.
Chen J, Brunner A-D, Cogan JZ, Nuñez JK, Fields AP, Adamson B, et al. Pervasive functional translation of noncanonical human open reading frames. Science. 2020;367:1140–6.
Zhang Q, Wu E, Tang Y, Cai T, Zhang L, Wang J, et al. Deeply mining a universe of peptides encoded by long noncoding RNAs. Mol Cell Proteom. 2021;20:100109.
Liu Q, Peng X, Shen M, Qian Q, Xing J, Li C, et al. Ribo-uORF:a comprehensive data resource of upstream open reading frames (uORFs) based on ribosome profiling. Nucleic Acids Res. 2023;51:D248–61.
Prensner JR, Enache OM, Luria V, Krug K, Clauser KR, Dempster JM, et al. Noncanonical open reading frames encode functional proteins essential for cancer cell survival. Nat Biotechnol. 2021;39:697–704.
Lee DSM, Park J, Kromer A, Baras A, Rader DJ, Ritchie MD, et al. Disrupting upstream translation in mRNAs is associated with human disease. Nat Commun. 2021;12:1515.
Slavoff SA, Mitchell AJ, Schwaid AG, Cabili MN, Ma J, Levin JZ, et al. Peptidomic discovery of short open reading frame–encoded peptides in human cells. Nat Chem Biol. 2013;9:59–64.
Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–5.
Niu L, Lou F, Sun Y, Sun L, Cai X, Liu Z, et al. A micropeptide encoded by lncRNA MIR155HG suppresses autoimmune inflammation via modulating antigen presentation. Sci Adv. 2020;6:eaaz2059.
Ho L, Tan SYX, Wee S, Wu Y, Tan SJC, Ramakrishna NB, et al. ELABELA Is an Endogenous Growth Factor that Sustains hESC Self-Renewal via the PI3K/AKT Pathway. Cell Stem Cell. 2015;17:435–47.
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–54.
Anderson DM, Anderson KM, Chang C-L, Makarewich CA, Nelson BR, McAnally JR, et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606.
Starck SR, Tsai JC, Chen K, Shodiya M, Wang L, Yahiro K, et al. Translation from the 5′ untranslated region shapes the integrated stress response. Science. 2016;351:aad3867.
Barczak W, Carr SM, Liu G, Munro S, Nicastri A, Lee LN, et al. Long non-coding RNA-derived peptides are immunogenic and drive a potent anti-tumour response. Nat Commun. 2023;14:1078.
Martinez TF, Lyons-Abbott S, Bookout AL, Souza EVD, Donaldson C, Vaughan JM, et al. Profiling mouse brown and white adipocytes to identify metabolically relevant small ORFs and functional microproteins. Cell Metab. 2023;35:166–83.e11.
Wu Y, Yang Y, Gu H, Tao B, Zhang E, Wei J, et al. Multi-omics analysis reveals the functional transcription and potential translation of enhancers. Int J Cancer. 2020;147:2210–24.
Chew G-L, Pauli A, Schier AF. Conservation of uORF repressiveness and sequence features in mouse, human and zebrafish. Nat Commun. 2016;7:11663.
Manske F, Ogoniak L, Jürgens L, Grundmann N, Makałowski W, Wethmar K. The new uORFdb:integrating literature, sequence, and variation data in a central hub for uORF research. Nucleic Acids Res. 2023;51:D328–36.
Samandi S, Roy AV, Delcourt V, Lucier J-F, Gagnon J, Beaudoin MC, et al. Deep transcriptome annotation enables the discovery and functional characterization of cryptic small proteins. eLife 2017;6:e27860.
Wu Q, Wright M, Gogol MM, Bradford WD, Zhang N, Bazzini AA. Translation of small downstream ORFs enhances translation of canonical main open reading frames. EMBO J. 2020;39:e104763.
Yang Y, Wang Z. IRES-mediated cap-independent translation, a path leading to hidden proteome. J Mol Cell Biol. 2019;11:911–9.
Chong C, Müller M, Pak H, Harnett D, Huber F, Grun D, et al. Integrated proteogenomic deep sequencing and analytics accurately identify non-canonical peptides in tumor immunopeptidomes. Nat Commun. 2020;11:1293.
Bazzini AA, Johnstone TG, Christiano R, Mackowiak SD, Obermayer B, Fleming ES, et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014;33:981–93.
Bencivenga D, Caldarelli I, Stampone E, Mancini FP, Balestrieri ML, Della Ragione F, et al. p27Kip1 and human cancers:A reappraisal of a still enigmatic protein. Cancer Lett. 2017;403:354–65.
Occhi G, Regazzo D, Trivellin G, Boaretto F, Ciato D, Bobisse S, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLOS Genet. 2013;9:e1003350.
Polycarpou-Schwarz M, Groß M, Mestdagh P, Schott J, Grund SE, Hildenbrand C, et al. The cancer-associated microprotein CASIMO1 controls cell proliferation and interacts with squalene epoxidase modulating lipid droplet formation. Oncogene. 2018;37:4750–68.
Guo Z-W, Meng Y, Zhai X-M, Xie C, Zhao N, Li M, et al. Translated long non-coding ribonucleic acid ZFAS1 promotes cancer cell migration by elevating reactive oxygen species production in hepatocellular carcinoma. Front Genet. 2019;10:1111.
Xu W, Deng B, Lin P, Liu C, Li B, Huang Q, et al. Ribosome profiling analysis identified a KRAS-interacting microprotein that represses oncogenic signaling in hepatocellular carcinoma cells. Sci China Life Sci. 2020;63:529–42.
Wang Y, Wu S, Zhu X, Zhang L, Deng J, Li F, et al. LncRNA-encoded polypeptide ASRPS inhibits triple-negative breast cancer angiogenesis. J Exp Med. 2019;217:e20190950.
Guo B, Wu S, Zhu X, Zhang L, Deng J, Li F, et al. Micropeptide CIP2A-BP encoded by LINC00665 inhibits triple-negative breast cancer progression. EMBO J. 2020;39:e102190.
Wu S, Zhang L, Deng J, Guo B, Li F, Wang Y, et al. A novel micropeptide encoded by Y-linked LINC00278 links cigarette smoking and AR signaling in male esophageal squamous cell carcinoma. Cancer Res. 2020;80:2790–803.
Meng N, Chen M, Chen D, Chen X, Wang J, Zhu S, et al. Small protein hidden in lncRNA LOC90024 promotes “cancerous” RNA splicing and tumorigenesis. Adv Sci. 2020;7:1903233.
Li M, Li X, Zhang Y, Wu H, Zhou H, Ding X, et al. Micropeptide MIAC inhibits HNSCC progression by interacting with aquaporin 2. J Am Chem Soc. 2020;142:6708–16.
Li XL, Pongor L, Tang W, Das S, Muys BR, Jones MF, et al. A small protein encoded by a putative lncRNA regulates apoptosis and tumorigenicity in human colorectal cancer cells. eLife. 2020;9:e53734.
Kang M, Tang B, Li J, Zhou Z, Liu K, Wang R, et al. Identification of miPEP133 as a novel tumor-suppressor microprotein encoded by miR-34a pri-miRNA. Mol Cancer. 2020;19:143.
Li L, Shu X, Geng H, Ying J, Guo L, Luo J, et al. A novel tumor suppressor encoded by a 1p36.3 lncRNA functions as a phosphoinositide-binding protein repressing AKT phosphorylation/activation and promoting autophagy. Cell Death Differ. 2023;30:1166–83.
Yang J-E, Zhong W-J, Li J-F, Lin Y-Y, Liu F-T, Tian H, et al. LINC00998-encoded micropeptide SMIM30 promotes the G1/S transition of cell cycle by regulating cytosolic calcium level. Mol Oncol. 2023;17:901–16.
Zheng C, Wei Y, Zhang P, Xu L, Zhang Z, Lin K, et al. CRISPR/Cas9 screen uncovers functional translation of cryptic lncRNA-encoded open reading frames in human cancer. J Clin Investig. 2023;133:e159940.
Jang C, Blume SW, Choi HS. Novel protein products encoded by upstream open reading frames of the MYCN gene in pediatric embryonal tumors. J Cell Biochem. 2023;124:1615–27.
Hofman DA, Ruiz-Orera J, Yannuzzi I, Murugesan R, Brown A, Clauser KR, et al. Translation of non-canonical open reading frames as a cancer cell survival mechanism in childhood medulloblastoma. Mol Cell. 2024;84:261–76.e18.
Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat Med. 2019;25:1488–99.
Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. 2023;8:1–38.
Carbonnelle D, Vignard V, Sehedic D, Moreau-Aubry A, Florenceau L, Charpentier M, et al. The melanoma antigens MELOE-1 and MELOE-2 are translated from a bona fide polycistronic mRNA containing functional IRES sequences. PLoS ONE. 2013;8:e75233.
Swoboda RK, Somasundaram R, Caputo-Gross L, Marincola FM, Robbins P, Herlyn M, et al. Antimelanoma CTL recognizes peptides derived from an ORF transcribed from the antisense strand of the 3′ untranslated region of TRIT1. Mol Ther Oncolytics. 2014;1:14009.
Jürgens L, Manske F, Hubert E, Kischka T, Flötotto L, Klaas O, et al. Somatic functional deletions of upstream open reading frame-associated initiation and termination codons in human cancer. Biomedicines. 2021;9:618.
Whiffin N, Karczewski KJ, Zhang X, Chothani S, Smith MJ, Evans DG, et al. Characterising the loss-of-function impact of 5’ untranslated region variants in 15,708 individuals. Nat Commun. 2020;11:2523.
Nelde A, Flötotto L, Jürgens L, Szymik L, Hubert E, Bauer J, et al. Upstream open reading frames regulate translation of cancer-associated transcripts and encode HLA-presented immunogenic tumor antigens. Cell Mol Life Sci. 2022;79:171.
Puig-Saus C, Sennino B, Peng S, Wang CL, Pan Z, Yuen B, et al. Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy. Nature. 2023;615:697–704.
Wells DK, van Buuren MM, Dang KK, Hubbard-Lucey VM, Sheehan KCF, Campbell KM, et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 2020;183:818–34.e13.
Boulias K, Greer EL. Biological roles of adenine methylation in RNA. Nat Rev Genet. 2023;24:143–60.
Flamand MN, Meyer KD. m6A and YTHDF proteins contribute to the localization of select neuronal mRNAs. Nucleic Acids Res. 2022;50:4464–83.
Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, Pickering BF, et al. m6A enhances the phase separation potential of mRNA. Nature. 2019;571:424–8.
Cheng Y, Xie W, Pickering BF, Chu KL, Savino AM, Yang X, et al. N6-Methyladenosine on mRNA facilitates a phase-separated nuclear body that suppresses myeloid leukemic differentiation. Cancer Cell. 2021;39:958–72.e8.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6.
Roundtree IA, Luo G-Z, Zhang Z, Wang X, Zhou T, Cui Y, et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife. 2017;6:e31311.
Uddin MB, Wang Z, Yang C. The m6A RNA methylation regulates oncogenic signaling pathways driving cell malignant transformation and carcinogenesis. Mol Cancer. 2021;20:61.
Cao X, Geng Q, Fan D, Wang Q, Wang X, Zhang M, et al. m6A methylation: a process reshaping the tumour immune microenvironment and regulating immune evasion. Mol Cancer. 2023;22:42.
Gu J, Zhan Y, Zhuo L, Zhang Q, Li G, Li Q, et al. Biological functions of m6A methyltransferases. Cell Biosci. 2021;11:15.
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:1–16.
Xiao W, Adhikari S, Dahal U, Chen Y-S, Hao Y-J, Sun B-F, et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–19.
Tanabe A, Tanikawa K, Tsunetomi M, Takai K, Ikeda H, Konno J, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016;376:34–42.
Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m6A transcripts by the 3ʹ→5ʹ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68:374–87.e12.
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–27.
Mao Y, Dong L, Liu X-M, Guo J, Ma H, Shen B, et al. m6A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun. 2019;10:5332.
Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–99.
Zhuang M, Li X, Zhu J, Zhang J, Niu F, Liang F, et al. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Res. 2019;47:4765–77.
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20.
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat Commun. 2016;7:12626.
Li A, Chen Y-S, Ping X-L, Yang X, Xiao W, Yang Y, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–7.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–28.
Li P, Shi Y, Gao D, Xu H, Zou Y, Wang Z, et al. ELK1-mediated YTHDF1 drives prostate cancer progression by facilitating the translation of Polo-like kinase 1 in an m6A dependent manner. Int J Biol Sci. 2022;18:6145–62.
Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–31.
Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6:1–13.
Wang S, Gao S, Zeng Y, Zhu L, Mo Y, Wong CC, et al. N6-methyladenosine reader YTHDF1 promotes ARHGEF2 translation and RhoA signaling in colorectal cancer. Gastroenterology. 2022;162:1183–96.
Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian S-B. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–4.
Coots RA, Liu X-M, Mao Y, Dong L, Zhou J, Wan J, et al. m6A facilitates eIF4F-independent mRNA translation. Mol Cell. 2017;68:504–14.e7.
Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5′ UTR m6A promotes cap-independent translation. Cell. 2015;163:999–1010.
Pi J, Wang W, Ji M, Wang X, Wei X, Jin J, et al. YTHDF1 promotes gastric carcinogenesis by controlling translation of FZD7. Cancer Res. 2021;81:2651–65.
Hong Y-G, Yang Z, Chen Y, Liu T, Zheng Y, Zhou C, et al. The RNA m6A reader YTHDF1 is required for acute myeloid leukemia progression. Cancer Res. 2023;83:845–60.
Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, et al. mRNA circularization by METTL3–eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–60.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–45.
Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, et al. YTHDF3 induces the translation of m6A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38:857–71.e7.
Benavides-Serrato A, Saunders JT, Kumar S, Holmes B, Benavides KE, Bashir MT, et al. m6A-modification of cyclin D1 and c-myc IRESs in glioblastoma controls ITAF activity and resistance to mTOR inhibition. Cancer Lett. 2023;562:216178.
Han SH, Choe J. Diverse molecular functions of m6A mRNA modification in cancer. Exp Mol Med. 2020;52:738–49.
Jia J, Wu S, Jia Z, Wang C, Ju C, Sheng J, et al. Novel insights into m6A modification of coding and non-coding RNAs in tumor biology: From molecular mechanisms to therapeutic significance. Int J Biol Sci. 2022;18:4432–51.
Chen Z, Zhong X, Xia M, Zhong J. The roles and mechanisms of the m6A reader protein YTHDF1 in tumor biology and human diseases. Mol Ther Nucleic Acids. 2021;26:1270–9.
Su R, Dong L, Li Y, Gao M, He PC, Liu W, et al. METTL16 exerts an m6A-independent function to facilitate translation and tumorigenesis. Nat Cell Biol. 2022;24:205–16.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677–91.e10.
Xiao Z, Li T, Zheng X, Lin L, Wang X, Li B, et al. Nanodrug enhances post-ablation immunotherapy of hepatocellular carcinoma via promoting dendritic cell maturation and antigen presentation. Bioact Mater. 2023;21:57–68.
Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96.e11.
Liu Y, Liang G, Xu H, Dong W, Dong Z, Qiu Z, et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33:1221–33.e11.
Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–84.
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–34.
Selberg S, Seli N, Kankuri E, Karelson M. Rational design of novel anticancer small-molecule RNA m6A demethylase ALKBH5 inhibitors. ACS Omega. 2021;6:13310–20.
Takahashi H, Hase H, Yoshida T, Tashiro J, Hirade Y, Kitae K, et al. Discovery of two novel ALKBH5 selective inhibitors that exhibit uncompetitive or competitive type and suppress the growth activity of glioblastoma multiforme. Chem Biol Drug Des. 2022;100:1–12.
Wang L, Dou X, Chen S, Yu X, Huang X, Zhang L, et al. YTHDF2 inhibition potentiates radiotherapy antitumor efficacy. Cancer Cell. 2023;41:1294–1308.e8.
Micaelli M, Dalle Vedove A, Cerofolini L, Vigna J, Sighel D, Zaccara S, et al. Small-molecule ebselen binds to YTHDF proteins interfering with the recognition of N6-methyladenosine-modified RNAs. ACS Pharmacol Transl Sci. 2022;5:872–91.
Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–101.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91.
Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188–206.
Cheng J, Li G, Wang W, Stovall DB, Sui G, Li D. Circular RNAs with protein-coding ability in oncogenesis. Biochim Biophys Acta BBA Rev Cancer. 2023;1878:188909.
Pisignano G, Michael DC, Visal TH, Pirlog R, Ladomery M, Calin GA. Going circular: history, present, and future of circRNAs in cancer. Oncogene. 2023;42:2783–800.
Stagsted LV, Nielsen KM, Daugaard I, Hansen TB. Noncoding AUG circRNAs constitute an abundant and conserved subclass of circles. Life Sci Alliance. 2019;2:e201900398.
Ho-Xuan H, Glažar P, Latini C, Heizler K, Haase J, Hett R, et al. Comprehensive analysis of translation from overexpressed circular RNAs reveals pervasive translation from linear transcripts. Nucleic Acids Res. 2020;48:10368–82.
Heesch, van S, Witte F, Schneider-Lunitz V, Schulz JF, Adami E, Faber AB, et al. The translational landscape of the human heart. Cell. 2019;178:242–60.e29.
Fan X, Yang Y, Chen C, Wang Z. Pervasive translation of circular RNAs driven by short IRES-like elements. Nat Commun. 2022;13:3751.
Li H, Xie M, Wang Y, Yang L, Xie Z, Wang H. riboCIRC:a comprehensive database of translatable circRNAs. Genome Biol. 2021;22:79.
Welden JR, Margvelani G, Arizaca Maquera KA, Gudlavalleti B, Miranda Sardón SC, Campos AR, et al. RNA editing of microtubule-associated protein tau circular RNAs promotes their translation and tau tangle formation. Nucleic Acids Res. 2022;50:12979–96.
Mo D, Li X, Raabe CA, Rozhdestvensky TS, Skryabin BV, Brosius J. Circular RNA encoded amyloid beta peptides—a novel putative player in Alzheimer’s disease. Cells. 2020;9:2196.
Du WW, Xu J, Yang W, Wu N, Li F, Zhou L, et al. A neuroligin isoform translated by circNlgn contributes to cardiac remodeling. Circ Res. 2021;129:568–82.
Guo J, Chen L-W, Huang Z-Q, Guo J-S, Li H, Shan Y, et al. Suppression of the inhibitory effect of circ_0036176-translated Myo9a-208 on cardiac fibroblast proliferation by miR-218-5p. J Cardiovasc Transl Res. 2022;15:548–59.
Sun W, Zhou S, Peng L, Liu Y, Cheng D, Wang Y, et al. CircZNF609 regulates pulmonary fibrosis via miR-145-5p/KLF4 axis and its translation function. Cell Mol Biol Lett. 2023;28:105.
Chen C-K, Cheng R, Demeter J, Chen J, Weingarten-Gabbay S, Jiang L, et al. Structured elements drive extensive circular RNA translation. Mol Cell. 2021;81:4300–18.e13.
Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–5.
Jang SK, Kräusslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 5’ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–43.
Macejak DG, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature. 1991;353:90–4.
Weingarten-Gabbay S, Elias-Kirma S, Nir R, Gritsenko AA, Stern-Ginossar N, Yakhini Z, et al. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science. 2016;351:aad4939.
Zhao J, Li Y, Wang C, Zhang H, Zhang H, Jiang B, et al. IRESbase: a comprehensive database of experimentally validated internal ribosome entry sites. Genomics Proteom Bioinform. 2020;18:129–39.
Haizel SA, Bhardwaj U, Gonzalez RL, Mitra S, Goss DJ. 5′-UTR recruitment of the translation initiation factor eIF4GI or DAP5 drives cap-independent translation of a subset of human mRNAs. J Biol Chem. 2020;295:11693–706.
Hundsdoerfer P, Thoma C, Hentze MW. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc Natl Acad Sci USA 2005;102:13421–6.
Nevins TA, Harder ZM, Korneluk RG, Holčı́k M. Distinct regulation of internal ribosome entry site-mediated translation following cellular stress is mediated by apoptotic fragments of eIF4G translation initiation factor family members eIF4GI and p97/DAP5/NAT1. J Biol Chem. 2003;278:3572–9.
Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–8.
Quinto SL, de, Lafuente E, Martínez-Salas E. IRES interaction with translation initiation factors:functional characterization of novel RNA contacts with eIF3, eIF4B, and eIF4GII. RNA 2001;7:1213–26.
Fraser CS, Doudna JA. Structural and mechanistic insights into hepatitis C viral translation initiation. Nat Rev Microbiol. 2007;5:29–38.
Thakor N, Smith MD, Roberts L, Faye MD, Patel H, Wieden H-J, et al. Cellular mRNA recruits the ribosome via eIF3-PABP bridge to initiate internal translation. RNA Biol. 2017;14:553–67.
Simon AE, Miller WA. 3′ Cap-independent translation enhancers of plant viruses. Annu Rev Microbiol. 2013;67:21–42.
Shatsky IN, Terenin IM, Smirnova VV, Andreev DE. Cap-independent translation: what’s in a name? Trends Biochem Sci. 2018;43:882–95.
Geng G, Wang D, Liu Z, Wang Y, Zhu M, Cao X, et al. Translation of plant RNA viruses. Viruses. 2021;13:2499.
Danthinne X, Seurinck J, Meulewaeter F, Montagu MV, Cornelissen M. The 3’ untranslated region of satellite tobacco necrosis virus RNA stimulates translation in vitro. Mol Cell Biol. 1993. https://doi.org/10.1128/mcb.13.6.3340-3349.1993.
Andreev DE, Dmitriev SE, Zinovkin R, Terenin IM, Shatsky IN. The 5′ untranslated region of Apaf-1 mRNA directs translation under apoptosis conditions via a 5′ end-dependent scanning mechanism. FEBS Lett. 2012;586:4139–43.
Terenin IM, Andreev DE, Dmitriev SE, Shatsky IN. A novel mechanism of eukaryotic translation initiation that is neither m7G-cap-, nor IRES-dependent. Nucleic Acids Res. 2013;41:1807–16.
Zhou Y, Wu J, Yao S, Xu Y, Zhao W, Tong Y, et al. DeepCIP: a multimodal deep learning method for the prediction of internal ribosome entry sites of circRNAs. Comput Biol Med. 2023;164:107288.
Godet A-C, David F, Hantelys F, Tatin F, Lacazette E, Garmy-Susini B, et al. IRES trans-acting factors, key actors of the stress response. Int J Mol Sci. 2019;20:924.
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27:626–41.
Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, et al. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–76.
Di Timoteo G, Dattilo D, Centrón-Broco A, Colantoni A, Guarnacci M, Rossi F, et al. Modulation of circRNA metabolism by m6A modification. Cell Rep. 2020;31:107641.
Zhong J, Wu X, Gao Y, Chen J, Zhang M, Zhou H, et al. Circular RNA encoded MET variant promotes glioblastoma tumorigenesis. Nat Commun. 2023;14:4467.
Duan J-L, Chen W, Xie J-J, Zhang M-L, Nie R-C, Liang H, et al. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:93.
Abe N, Matsumoto K, Nishihara M, Nakano Y, Shibata A, Maruyama H, et al. Rolling circle translation of circular RNA in living human cells. Sci Rep. 2015;5:16435.
Abe N, Hiroshima M, Maruyama H, Nakashima Y, Nakano Y, Matsuda A, et al. Rolling circle amplification in a prokaryotic translation system using small circular RNA. Angew Chem Int Ed. 2013;52:7004–8.
Perriman R, Ares M. Circular mRNA can direct translation of extremely long repeating-sequence proteins in vivo. RNA. 1998;4:1047–54.
AbouHaidar MG, Venkataraman S, Golshani A, Liu B, Ahmad T. Novel coding, translation, and gene expression of a replicating covalently closed circular RNA of 220 nt. Proc Natl Acad Sci USA 2014;111:14542–7.
Liu Y, Li Z, Zhang M, Zhou H, Wu X, Zhong J, et al. Rolling-translated EGFR variants sustain EGFR signaling and promote glioblastoma tumorigenicity. Neuro Oncol. 2021;23:743–56.
Das A, Sinha T, Mishra SS, Das D, Panda AC. Identification of potential proteins translated from circular RNA splice variants. Eur J Cell Biol. 2023;102:151286.
Chang J, Shin M-K, Park J, Hwang HJ, Locker N, Ahn J, et al. An interaction between eIF4A3 and eIF3g drives the internal initiation of translation. Nucleic Acids Res. 2023;51:10950–69.
Lin H-H, Chang C-Y, Huang Y-R, Shen C-H, Wu Y-C, Chang K-L, et al. Exon junction complex mediates the cap-independent translation of circular RNA. Mol Cancer Res. 2023;21:1220–33.
Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P, et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9:4475.
Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37:1805–14.
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. JNCI. 2018;110:304–15.
Xia X, Li X, Li F, Wu X, Zhang M, Zhou H, et al. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol Cancer. 2019;18:131.
Liang Z, Liu H, Xiong L, Yang X, Wang F, Zeng Z, et al. A novel NF-κB regulator encoded by circPLCE1 inhibits colorectal carcinoma progression by promoting RPS3 ubiquitin-dependent degradation. Mol Cancer. 2021;20:103.
Pan Z, Cai J, Lin J, Zhou H, Peng J, Liang J, et al. A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating Snail in colon cancer. Mol Cancer. 2020;19:71.
Wang L, Zhou J, Zhang C, Chen R, Sun Q, Yang P, et al. A novel tumour suppressor protein encoded by circMAPK14 inhibits progression and metastasis of colorectal cancer by competitively binding to MKK6. Clin Transl Med. 2021;11:e613.
Li K, Peng Z-Y, Wang R, Li X, Du N, Liu D-P, et al. Enhancement of TKI sensitivity in lung adenocarcinoma through m6A-dependent translational repression of Wnt signaling by circ-FBXW7. Mol Cancer. 2023;22:103.
Zhang Y, Jiang J, Zhang J, Shen H, Wang M, Guo Z, et al. CircDIDO1 inhibits gastric cancer progression by encoding a novel DIDO1-529aa protein and regulating PRDX2 protein stability. Mol Cancer. 2021;20:101.
Li Y, Chen B, Zhao J, Li Q, Chen S, Guo T, et al. HNRNPL circularizes ARHGAP35 to produce an oncogenic protein. Adv Sci. 2021;8:2001701.
Lu J, Ru J, Chen Y, Ling Z, Liu H, Ding B, et al. N 6‐methyladenosine‐modified circSTX6 promotes hepatocellular carcinoma progression by regulating the HNRNPD/ATF3 axis and encoding a 144 amino acid polypeptide. Clin Transl Med. 2023;13:e1451.
Song R, Ma S, Xu J, Ren X, Guo P, Liu H, et al. A novel polypeptide encoded by the circular RNA ZKSCAN1 suppresses HCC via degradation of mTOR. Mol Cancer. 2023;22:16.
Gu C, Zhou N, Wang Z, Li G, Kou Y, Yu S, et al. circGprc5a promoted bladder oncogenesis and metastasis through Gprc5a-targeting peptide. Mol Ther Nucleic Acids. 2018;13:633–41.
Wang X, Jian W, Luo Q, Fang L. CircSEMA4B inhibits the progression of breast cancer by encoding a novel protein SEMA4B-211aa and regulating AKT phosphorylation. Cell Death Dis. 2022;13:1–16.
Vaklavas C, Meng Z, Choi H, Grizzle WE, Zinn KR, Blume SW. Small molecule inhibitors of IRES-mediated translation. Cancer Biol Ther. 2015;16:1471–85.
Komar AA, Hatzoglou M. Exploring internal ribosome entry sites as therapeutic targets. Front Oncol. 2015;5:233.
Marques R, Lacerda R, Romão L. Internal ribosome entry site (IRES)-mediated translation and its potential for novel mRNA-based therapy development. Biomedicines. 2022;10:1865.
Benavides-Serrato A, Saunders JT, Holmes B, Nishimura RN, Lichtenstein A, Gera J. Repurposing potential of riluzole as an ITAF inhibitor in mTOR therapy resistant glioblastoma. Int J Mol Sci. 2020;21:344.
Zhang F, Jiang J, Qian H, Yan Y, Xu W. Exosomal circRNA:emerging insights into cancer progression and clinical application potential. J Hematol Oncol. 2023;16:67.
Loan Young T, Chang Wang K, James Varley A, Li B. Clinical delivery of circular RNA:Lessons learned from RNA drug development. Adv Drug Deliv Rev. 2023;197:114826.
Li H, Peng K, Yang K, Ma W, Qi S, Yu X, et al. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics. 2022;12:6422–36.
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z, et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24:357–70.
Yi Z, Li Y, Wu Y, Zeng B, Li H, Ren G, et al. Circular RNA 0001073 attenuates malignant biological behaviours in breast cancer cell and is delivered by nanoparticles to inhibit mice tumour growth. OncoTargets Ther. 2020;13:6157–69.
Wu X, Xiao S, Zhang M, Yang L, Zhong J, Li B, et al. A novel protein encoded by circular SMO RNA is essential for Hedgehog signaling activation and glioblastoma tumorigenicity. Genome Biol. 2021;22:33.
Uribe ML, Marrocco I, Yarden Y. EGFR in cancer: signaling mechanisms, drugs, and acquired resistance. Cancers. 2021;13:2748.
Gao X, Xia X, Li F, Zhang M, Zhou H, Wu X, et al. Circular RNA-encoded oncogenic E-cadherin variant promotes glioblastoma tumorigenicity through activation of EGFR–STAT3 signalling. Nat Cell Biol. 2021;23:278–91.
Li J, Ma M, Yang X, Zhang M, Luo J, Zhou H, et al. Circular HER2 RNA positive triple negative breast cancer is sensitive to Pertuzumab. Mol Cancer. 2020;19:142.
Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z, Xu B, et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer. 2019;18:47.
Liang W-C, Wong C-W, Liang P-P, Shi M, Cao Y, Rao S-T, et al. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20:84.
Li Y, Wang Z, Su P, Liang Y, Li Z, Zhang H, et al. circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol Ther. 2022;30:415–30.
Peng Y, Xu Y, Zhang X, Deng S, Yuan Y, Luo X, et al. A novel protein AXIN1-295aa encoded by circAXIN1 activates the Wnt/β-catenin signaling pathway to promote gastric cancer progression. Mol Cancer. 2021;20:158.
Somasekharan SP, Saxena N, Zhang F, Beraldi E, Huang JN, Gentle C, et al. Regulation of AR mRNA translation in response to acute AR pathway inhibition. Nucleic Acids Res. 2022;50:1069–91.
Jiang T, Xia Y, Lv J, Li B, Li Y, Wang S, et al. A novel protein encoded by circMAPK1 inhibits progression of gastric cancer by suppressing activation of MAPK signaling. Mol Cancer. 2021;20:66.
Saunders JT, Kumar S, Benavides-Serrato A, Holmes B, Benavides KE, Bashir MT, et al. Translation of circHGF RNA encodes an HGF protein variant promoting glioblastoma growth through stimulation of c-MET. J Neurooncol. 2023;163:207–18.
Gu C, Wang W, Tang X, Xu T, Zhang Y, Guo M, et al. CHEK1 and circCHEK1_246aa evoke chromosomal instability and induce bone lesion formation in multiple myeloma. Mol Cancer. 2021;20:84.
Yang F, Hu A, Guo Y, Wang J, Li D, Wang X, et al. p113 isoform encoded by CUX1 circular RNA drives tumor progression via facilitating ZRF1/BRD4 transactivation. Mol Cancer. 2021;20:123.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (2042022dx0003) and the National Natural Science Foundation of China (32070832 and 32150610476).
Author information
Authors and Affiliations
Contributions
XD: conceptualization, writing - original draft, visualization; YVY: writing – review & editing, project administration; YNJ: conceptualization, writing - original draft, visualization, writing – review & editing, project administration, supervision, funding acquisition. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have approved to publish this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, X., Yu, Y.V. & Jin, Y.N. Non-canonical translation in cancer: significance and therapeutic potential of non-canonical ORFs, m6A-modification, and circular RNAs. Cell Death Discov. 10, 412 (2024). https://doi.org/10.1038/s41420-024-02185-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-024-02185-y
This article is cited by
-
Noncoding RNA-encoded peptides in cancer: biological functions, posttranslational modifications and therapeutic potential
Journal of Hematology & Oncology (2025)
-
N7-methylguanosine modification in cancers: from mechanisms to therapeutic potential
Journal of Hematology & Oncology (2025)
-
Cytoskeletal protein KRT14 governs cisplatin resistance by modulating eIF4H-dependent ACOX2 translation and lipid metabolism in bladder cancer
Cell Death & Disease (2025)