Abstract
Colorectal cancer (CRC) is one of the most common tumors in the digestive system, and the majority of patients are found to be in advanced stages, which is a burden to human health all over the world. Moreover, in recent years, CRC has been progressively becoming younger, with an increasing incidence mainly among patients <50 years old. Despite the increase in awareness of CRC and the continuous improvement of medical treatment nowadays, the challenge of CRC still needs to be conquered. By now, the pathogenesis of CRC is complex and not fully understood. With the deepening of research, it has been revealed that PPARs, as a transcription factor, are inextricably linked to CRC. This article outlines the mechanisms by which PPARs are involved in CRC development. An in-depth understanding of the pathways related to PPARs may provide new ways of developing effective therapies for CRC with PPARs as potential targets.
Similar content being viewed by others
Facts
-
PPAR is a nuclear receptor that exists in three subtypes: PPARα, PPARβ /δ, and PPARγ. They function as transcription factors and can participate in tumorigenesis, cell proliferation, angiogenesis, cell death, invasion, migration, and tumor metabolic processes.
-
Most current studies have shown that PPARα and PPARβ /δ are highly expressed, while PPARγ is suppressed in CRC.
-
Although the role of PPARs in CRC is not fully understood, developing novel therapies targeting PPARs has become a new trend.
Open questions
-
What are all the mechanisms by which PPARs affect CRC progression through the tumor microenvironment?
-
Is targeted therapy for PPAR universal, and does this need to be validated by more clinical results?
-
Can PPAR expression levels be used as a screening and prognostic indicator for CRC and indicate patient condition?
-
Can PPAR modulators be used in combination with each other and with other therapies, and is there consistency in the role of their results in CRC?
Background
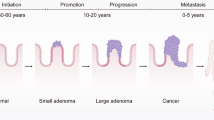
Colorectal cancer (CRC) represents the second most common cause of mortality worldwide. Many physiological and pathological mechanisms, including cell proliferation, angiogenesis, cell death, invasion and metastasis, and tumor metabolism, regulate the development and progression of CRC [1]. In recent years, researchers have made progress in CRC through continuous research. For example, they have identified many new diagnostic biomarkers and therapeutic targets that positively affect the diagnosis and treatment of CRC patients [2,3,4,5]. However, the incidence of CRC in patients <50 years of age has risen globally over the past decade. CRC has become the third leading cause of cancer deaths in people under 50 years of age. This rising trend may be related to risk factors [6, 7]. Common risk factors for CRC include genetic susceptibility, diet, obesity, alcohol consumption, infections, and lifestyle changes [1, 8,9,10]. Despite significant advancements in the diagnosis and surgical treatment of CRC, the occurrence of metastasis and recurrence continues to reduce patient survival rates [11, 12]. These reasons urge us to conduct further in-depth studies on CRC’s relevant transcriptional modes and pathways. This will facilitate the identification of efficacious targets for the prevention and treatment of cancer.
Transcription of PPAR
PPAR is an endogenous or exogenous ligand-activated transcription factor belonging to the nuclear hormone receptor superfamily. It currently exists in mammals in the form of three subtypes, including PPARα, PPARβ/δ, and PPARγ [13]. Binding of PPARs to ligands prompts binding to co-repressor proteins and heterodimerization with the retinoic acid X receptor. During this process, the protein conformation is altered, releasing co-repressors (e.g., SMRT and NCoR) and recruiting co-activators (e.g., p300, SRC-1). This process involves many transcriptional co-activator interactions, including PPARγ co-activator-1 (PGC-1) α and PGC-1β. The binding of this complex to the PPRE consensus DNA sequence (AGGTCANAGGTCA) in the promoter of the target gene, thereby regulating the transcription of the target gene. It regulates gene expression and cellular functions and is distributed in different tissues to perform biological functions [14,15,16,17].
Classification and physiological significance of PPAR
PPARα is the first subtype to be discovered [18]. It primarily regulates lipid and glucose dynamic homeostasis, the mobilization and catabolism of fatty acids, and the enhancement of fatty acid oxidation (FAO). PPARα is highly expressed in the liver, kidney, intestine, heart, skeletal muscle, and brown adipose tissue [18,19,20]. PPARβ/δ is widely distributed in various tissues, with the highest expression levels observed in colonic epithelial cells, skin, and adipocytes. The primary functions of PPARβ/δ are the regulation of glucose and lipid homeostasis, cellular energy expenditure, and blood lipid levels. Additionally, it stimulates lipogenesis, facilitates wound healing, and enhances resistance to adverse environmental conditions [19,20,21,22]. PPARγ has been extensively studied, and PPARγ is expressed in kidney and intestinal mucosa and white and brown adipose tissue. It is a primary regulator of adipocyte generation and differentiation and a key regulatory factor of lipid metabolism [17]. In addition to this, it is implicated in developmental processes. Given the involvement of different promoters in the coding process, PPARγ gives rise to two main subtypes: PPARγ1 and PPARγ2. PPARγ1 is expressed in numerous tissues, whereas PPARγ2 is solely expressed in adipose tissues but can be induced by high-fat diets (HFDs) in other tissues [21]. PPARγ is more inclined to exert favorable human anticancer effects [23].
PPAR plays a crucial role in regulating lipid metabolism, glucose metabolism, and the control of energy homeostasis. Moreover, it is also involved in other biological processes, including cell proliferation, differentiation, apoptosis, and angiogenesis [24, 25]. PPAR has been implicated in a wide range of human diseases. The role in developing certain diseases such as atherosclerosis, inflammation, immunity, cancer, and various metabolic disorders like obesity and diabetes mellitus is well-established [19, 24].
PPAR in CRC
Dysregulation of lipid metabolism is one of the important pathways for cancer formation and progression, and it is a key factor in the acquisition of energy supply by cancer cells to regulate the tumor environment [26]. One of the most critical functions of PPAR is maintaining lipid metabolic homeostasis and energy levels. Therefore, the PPAR signaling pathway is involved in the regulation of the complex network of cancer and has an intricate relationship with tumors. PPAR plays a noteworthy role in controlling cancer growth [27]. In CRC, with tumor progression, increased expression of PPARα and PPARβ/δ or decreased expression of PPARγ [24]. Thus, PPAR may have potential pharmacological significance in colon cancer [28]. Nevertheless, the function of PPAR in CRC remains incompletely elucidated. PPAR may serve as a tumor suppressor or a tumor-promoting factor, exerting antitumor or pro-tumor effects, respectively. The role of PPAR in CRC remains inconclusive due to the inconsistency of results observed across different animal models and human tissues. This variability is likely attributed to the influence of internal and external environmental factors [18, 29].
Cancer has a complex and precise ecosystem network containing tumor and non-tumor components. The interactions and crosstalk mechanisms between the two promote the development and progression of solid tumors [30, 31]. Cancer is characterized by unlimited cell proliferation and cell cycle imbalance [32]. The development of tumors is not a single process involving complex multi-mechanistic pathways. The operation of these pathways and mechanisms allows PPARs to play an important role in “cancer hallmarks [33].” This article will describe the mechanism and role of PPARs in CRC in several ways using the framework of the “cancer hallmarks” defined by Hanahan and Weinberg.
Mechanism and role of PPARα in CRC
Tumorigenesis and proliferation
The current study suggests that PPARα activation has an antiproliferative effect [34, 35]. It has been shown that PPARα deficiency enhances tumorigenicity in mice by mediating the RB1/E2F pathway to increase DNA methyltransferase 1 (DNMT1)-mediated p21 methylation and protein arginine methyltransferase 6 (PRMT6)-mediated p27 methylation. When the PPARα agonist fenofibrate was administered, this was reversed and inhibited colon carcinogenesis by suppressing intestinal cell proliferation in mice [34]. Notably, the PPAR pan-agonist bezafibrate increased the proliferation and survival of tumor-reactive CD8+ T cells; it also enhanced the inhibition effect of colon cancer development in mice when combined with PD-1 blockade [36]. The data presented clearly indicate that PPARα plays a crucial role in the occurrence and development of CRC. It can be reasonably deduced that PPARα agonists may be promising drugs for treating CRC [34]. In contrast, a study demonstrated that PPARα antagonists exhibited antiproliferative effects on paraganglioma, pancreatic, and CRC cells. This indicates that PPARα antagonists may possess anticancer potential [37].
A recent study has shown that the function and characterization of cancer stem cells (CSCs), which are the leading cause of cancer recurrence and drug resistance, mainly depends on methionine. SIRT1/PGC-1α/PPAR-α regulation during methionine deprivation is involved in the impaired stemness of cancer cells, decreasing the self-renewal and differentiation potential of CSCs. Consequently, this mechanism provides a foundation for regenerative medicine and the treatment of cancer recurrence [38]. The regulation of CSCs represents a crucial aspect of tumorigenesis. Moreover, an imbalance in CSCs is associated with malignant tumors’ advancement and developmental potential. The PPAR has been identified as a pivotal factor in the growth of CSCs, as evidenced by numerous studies [39].
Angiogenesis
Early studies have shown that PPARα agonists reduce transcriptional activation of COX-2 and VEGF, which are associated with tumor angiogenesis [40]. It has been described that the anti-angiogenic effect of pomegranate peel extract (PPE) is partly attributed to the activation of PPARα and PPARγ in human umbilical vein endothelial cells. Moreover, their antagonists can counteract PPE’s positive tumor angiogenesis inhibitory effects [41]. Although the anti-angiogenic effect of PPARα is well documented, some studies have demonstrated that it can also promote tumor angiogenesis [25, 42, 43]. This discrepancy based on the effects of different PPARα agonists in different animal models urges us to investigate further the mechanisms and outcomes of PPARα affecting angiogenesis in CRC.
Cell death
Early experiments have indicated that the activation of PPARα plays a role in the apoptotic process in colon cancer [44]. Experiments conducted by Gao et al. using human SW480, HCT-116, and other cells suggested that PPARα, an E3 ligase, induces Bcl2 ubiquitination and degradation, leading to apoptosis [45]. Mesenchymal stem cells-derived extracellular vesicles harboring miR-378a-3p have been reported to mediate the GATA2/AQP4/PPAR-α signaling pathway. It inhibits apoptosis and inflammatory bowel disease (IBD) in the mouse colonic epithelial cell line M064 through PPARα inactivation [46]. There is growing evidence that CRC is one of the most severe consequences of IBD and that IBD is involved in the development of CRC [47, 48]. The down-regulation of carnitine palmitoyltransferase-1A (CPT1A), a pivotal enzyme in FAO, reduces intestinal inflammation, oxidative stress, and apoptosis through inhibition of PPARα expression in dextran sulfate sodium (DSS)-induced HT-29 cells. This result suggests that PPARα plays an essential protective role in ulcerative colitis [49]. It can, therefore, be posited that PPARα may play a role in regulating the development of CRC by influencing the process of IBD.
Invasion and metastasis
N-acylethanolamine acid amidase inhibitor AM9053 inhibits tumor cell growth, proliferation, and migration via PPARα and TRPV1 in CRC cells. However, this antitumor effect can be disrupted by PPAR-α and TRPV1 antagonists [50]. Studies have shown that fenofibrate, as a PPARα agonist, is believed to reduce the methylation of the tumor suppressor gene CDKN2A by inhibiting the content and activity of DNMT1. Then, it regulates the cell cycle, promoting apoptosis and inhibiting cell proliferation via the RB/E2F pathway. Fenofibrate also reduces cancer cells’ invasion and migration ability [35]. In contrast, unlike the above findings, one mechanism promoting metastasis in ovarian cancer is the activation of the PI3K/Akt/NF-κB pathway by low doses of mono(2-ethylhexyl) phthalate in a PPARα-dependent manner. In a mouse model, the PPARα inhibitor GW6471 inhibited this metastatic effect [51]. These findings indicate a need for further exploration of CRC invasion and migration.
Tumor metabolism
Fibrate drugs, which belong to the class of PPARα agonists, can inhibit triglyceride synthesis by promoting β-oxidation, thereby modulating widely altered lipid signaling in cancer cells and impacting colon cancer survival and proliferation [52]. PPARα agonist Wy14,643 reduces CRC cell growth through inhibitory effects on Glut1 transcriptional activity, glucose uptake, and mTOR pathway [53]. Interestingly, a study demonstrated that increased bile acids lead to impaired Lgr5+ intestinal stem cell (ISC) function by inhibiting PPARα-mediated FAO. This may exacerbate the progression of colitis and promote colitis-associated colon cancer [54]. Nevertheless, some evidence suggests that PPARα may also exert a pro-cancer effect. Acid-adapted colon cancer cells have been observed to reorganize cancer cell metabolism by increasing PPARα activity and participate to some extent in cancer cell proliferation and invasion. Consequently, by exploiting the sensitivity of cancer cells to PPARα inhibition, PPARα inhibitors can impede this metabolic process for antitumor purposes [55]. Additionally, it has been shown that in a mouse colon cancer model, PPARα-deficient mice exhibit reduced tumor growth rates compared to wild-type mice. These PPARα-deficient mice improve the function of dendritic cells (DCs) by inhibiting PPARα, exerting a powerful antitumor effect. In other words, PPARα positively responded to the tumor-derived exosomes-mediated increase in lipid levels, which induced lipid droplets generation and FAO enhancement, resulting in DC immune dysfunction. Therefore, they suggested that inhibition of PPARα may also serve as one of the strategies for antitumor therapy [56]. However, more experimental findings are needed to support this. Figure 1 shows the role of PPARα in CRC.
Mechanism and role of PPARβ/δ in CRC
Tumorigenesis and proliferation
Less is known about PPARβ/δ than about the other two subtypes. The first evidence linking PPARβ/δ to cancer was found in CRC [57]. A study showed that elevated PPARβ/δ promotes colon tumorigenesis in an experimental mouse model [58]. Moreover, in subsequent studies, other investigators have demonstrated that colonic epithelial PPARβ/δ overexpression positively affects mice’s susceptibility to colon cancer [59]. They elucidated that PPARβ/δ overexpression in colon epithelial cells significantly enhances colon cancer susceptibility by promoting the expression of pro-tumorigenic genes, including IFITM3 and NRG1 [59]. In addition, Overexpression of PPARβ/δ also increases the incidence rate of colitis-associated colorectal cancer by mediating IL-6/activator of transcription 3 (STAT3) signaling to promote the expression of tumor-promoting genes, such as Notch3 and MUC1 [60]. However, some previous groups have obtained opposing results, with PPARβ/δ expression inhibiting CRC development and thus negatively regulating cancer progression [24, 61,62,63]. Along the same lines, other studies have shown that PPARβ/δ knockout mice have an increased risk of colon cancer carcinogenesis [64]. In the same way, the +294T/C (Rs2016520) SNP of PPARβ/δ, a downstream target gene of the Wnt/β-catenin signaling pathway, reduced the risk of CRC in a Mexican population [65]. The function of PPARβ/δ in the context of cancer remains a topic of contention within the scientific community. Given the inconclusive nature of the experimental results, using PPARβ/δ modulators in clinical practice cannot be recommended with certainty and should be cautiously undertaken.
Angiogenesis
PPARβ/δ has a more pronounced pro-angiogenic function compared to PPARα and PPARγ [43]. A previous study showed that PPARβ/δ facilitates endothelial cell proliferation and angiogenesis by inducing the expression of the pro-angiogenic cytokine IL-8 through transcriptional and post-transcriptional mechanisms [66, 67]. Consistent with this description, subsequent studies have further demonstrated that the increase in IL-8 mediated by high PPARβ/δ expression promotes tumor angiogenesis and metastasis formation [68]. Findings on PPARβ/δ activation inducing endothelial cell proliferation and angiogenesis through a VEGF-dependent mechanism have been demonstrated [69]. Wagner and colleagues verified the effect of PPARβ/δ on angiogenesis. Using a vascular-specific overexpression PPARβ/δ transgenic mouse model, the authors concluded that PPARβ/δ promotes tumor angiogenesis, growth, and metastasis through activation of the PDGF/PDGFR, c-Kit, and VEGF/VEGFR pathways [70]. In subsequent experiments, they used RNA sequencing to demonstrate this conclusion [25]. In the HCT-116 CRC cell line and mouse colon tumor epithelial cells, GW501516 activated PPARβ/δ, which further induced an increase in the expression of COX-2 and its derivative PGE2 and led to an elevated expression of pro-inflammatory mediators in the colonic mucosa, including CXCL1, CCL2, CCL3, CCL4, and IL-1β. PPARβ/δ via this pathway is involved in tumorigenesis [63]. Interestingly, PGE2 also can promote colorectal tumor growth by transactivating PPARβ/δ [71]. Hence, there is positive feedback between PPARβ/δ, COX-2, and PGE2, which is a key factor in promoting tumorigenesis [72]. Furthermore, the expression of PPARβ/δ and COX-2 in CRC may promote angiogenesis and the risk of venous vessel invasion [73].
Cell death
In 1999, He et al. initially demonstrated that adenomatous polyposis coli (APC) inhibits PPARβ/δ expression by interfering with the β-catenin/Tcf-4 pathway by analyzing whole gene expression profiles. Furthermore, alterations in this pathway, such as APC /β-catenin mutations, can increase PPARβ/δ activity. The nonsteroidal anti-inflammatory drug (NSAID) sulindac is thought to promote apoptosis in CRC cells by inhibiting PPARβ/δ expression [74]. Twenty years ago, the PPARβ/δ ligand GW501516 was validated in Apc(min) mice. This experiment established that activation of the anti-apoptotic pathway in intestinal epithelial cells by PPARβ/δ promotes intestinal tumor growth in mice [75]. Later, Liou and his colleagues found that PPARβ/δ can rescue apoptosis in CRC cells induced by inhibition of 14-3-3ε via NSAIDs such as sulindac. This further confirmed the role of PPARβ/δ in apoptosis [76]. The effect of NSAIDs on apoptosis always involves PPARβ/δ. 15-lipoxygenase-1 (15-LOX-1) expression is associated with NSAIDs, and its main product of metabolism, 13-S-hydroxyoctadecadienoic acid (13-S-HODE), promotes the apoptotic pathway by down-regulating PPARβ/δ expression [77]. A fascinating study found that high expression or activation of PPARβ/δ resisted the PPARγ-induced apoptosis effects in CRC cells. This resistance was mediated by survivin and caspase-3 [78].
Invasion and metastasis
High expression of PPARβ/δ in colorectal, lung, and breast cancers affects metastasis-free survival of patients. This finding implies that PPARβ/δ regulates cancer metastasis [68]. Notably, when on an HFD, PPARβ/δ activation later directly binds to the Nanog promoter, increasing Nanog expression, which induces the amplification of CSCs and promotes hepatic metastasis of CRC. In contrast, the knockdown of PPARβ/δ attenuated this metastasis-promoting effect and inhibited tumor development. This finding is consistent with the pathway by which the PPARβ/δ agonist GW501516 promotes CRC metastasis [57]. Beyond this, AMPK-induced PPARδ-S50 phosphorylation inhibits colon cancer growth and metastasis by suppressing the transcriptional activity of PPARδ [79].
Tumor metabolism
It has been proven that the transcription factor MYC inversely regulates the expression of genes in the WNT signaling pathway. MYC promotes the interaction between LEF1 and β-catenin by directly targeting LEF1, activating the expression of PPARβ/δ and Acyl CoA dehydrogenase 9(ACAD9). This process ultimately results in metabolic reprogramming in colon cancer cells [80]. Impressively, fibroblasts deficient in PPARβ/δ in CRC cells regulate epithelial oxidative responses, reduce oxidative stress, and block the cell cycle. These mechanisms would reduce intestinal tumor load and decrease and delay CRC development [81]. Mana et al. showed that HFD activates FAO metabolism in ISCs via PPARβ/δ and PPARα, enhancing the tumorigenic potential. In addition, The PPARβ/δ agonist gw501516 has the ability to upregulate FAO-related genes [82]. Furthermore, HFDs and agonists increase the number of ISCs by upregulating PPARβ/δ levels, which is linked to the initiation and progression of CRC [83]. It has been proposed that the PPARβ/δ agonist GW501516 may contribute to the development of colitis-associated colon carcinogenesis, potentially through the increased expression of Glu1h and SLC1A5 [22]. This result is consistent with previous reports by these authors that PPARβ/δ promotes cancer cell proliferation and tumor progression through activation of Glut1 and SLC1A5 transcription [84]. These facts confirm that PPARβ/δ promotes metabolic signaling in tumors. In comparison, the AMPK agonist metformin inhibits GW501516-induced expression of Glut1 and SLC1A5 [85]. Besides, epidermal growth factor receptor (EGFR)-mediated phosphorylation of PPARδ-Y108 increased the stability of PPARβ/δ through heat shock protein 90(HSP90) and promoted the transcription of Glu1 and SLC1A5. This, in turn, regulates cancer cell proliferation and metabolism [86]. In HCT-116 cells, hypoxic stress induces transcriptional activation of PPARβ/δ. p300 and PI3K/Akt pathways may play a role in regulating PPARβ/δ transactivation under such hypoxic conditions. As a result, PPARβ/δ deficiency has been shown to inhibit the development of colon cancer in a hypoxic environment [87]. Figure 2 summarizes the role of PPARβ/δ in CRC.
Mechanism and role of PPARγ in CRC
Tumorigenesis and proliferation
Previous experiments have shown that PPARγ gene and protein expression is higher in rodent colon tumors than controls, suggesting a tumorigenic role for PPARγ [88]. Some studies have demonstrated that PPARγ activation increases the formation of colon polyps and promotes CRC [89, 90]. However, this view has changed. Most believe PPARγ activation can inhibit cancer development through various mechanisms [91]. Most of the findings indicate that PPARγ plays a significant place in inhibiting tumor proliferation, including colon cancer [9, 32, 91]. For example, the PPARγ agonist 15-d-PGJ2 suppresses tumor cytokine expression by inhibiting the NF-kB pathway in Caco-2 and HT-29 colon cancer cell lines. In addition, rosiglitazone, another agonist of PPARγ, was found to impede colorectal cell proliferation by activating PTEN (a tumor suppressor) and regulating the expression of phosphatidylinositol 3-kinase (PI3-kinase) [23]. On the other hand, HOXC11, an oncogene, may promote cell proliferation in colon cancer and renal clear cell carcinoma by down-regulating PPARγ signaling [92]. Promising evidence that PPARγ agonists prevent the survival of colon CSCs offers a new strategy for controlling cancer progression [32]. These findings open up potential possibilities for treating CRC with PPARγ agonists. The roles and mechanisms of the three PPAR agonists in CRC are shown in Table 1.
Angiogenesis
It is widely acknowledged that PPARγ activation facilitates the inhibition of tumor angiogenesis [43]. The tumor microenvironment (TME), which encompasses tumor cells and cancer‑associated fibroblasts, interstitial tissues, blood vessels, and a variety of inflammatory and immune cells, chemokines, and cytokines, is a complex and not yet fully understood system [93]. An excellent article confirms that overcoming TME can increase the sensitivity of Chimeric antigen receptor (CAR) T cells [94]. Subsequent reports have corroborated the assertion that heightened awareness of TME, the most significant obstacle, will enhance the efficacy of CAR T cell therapy in solid tumors [95]. It is worth noting that angiogenesis in TME is a crucial element influencing cancer progression. Based on mouse models constructed from the colon cancer cell line CT26 and breast cancer cell line 4T1, the PPARγ agonist rosiglitazone was found to remodel the tumor vasculature system and limit tumor-associated macrophage (TAM) infiltration. It also exerted anti-angiogenic effects in vitro by inhibiting the VEGF/VEGFR2 signaling pathway. The authors finally found that combining rosiglitazone with radiotherapy effectively inhibited angiogenesis, distant metastatic potential, and tumor recurrence [96].
Cell death
It has been documented that the endogenous ligand 13(S)hydroperoxy octadecadienoic acid [13(S)HpODE] plays a role in colorectal tumor cell apoptosis through the MAO-A > ROS > P53 > p21 axis upon activation of PPARγ [97]. Chiral phenoxyacetic acid analog (S)-3 acts as a partial agonist of PPARγ, blocks the cell cycle, inhibits cell proliferation, and induces apoptosis through PPAR-dependent mechanisms: upregulation of p21waf1/cip1, and inhibition of c-Myc and cyclin D1 [98]. On the other hand, it has been reported that the PPARγ natural agonist, cladosporols, inhibits adipogenesis in vitro and suppresses tumor proliferation and invasive migration while promoting apoptosis. This mechanism of action provides a potential means of interfering with colon cancer growth [17]. In HT-29 human CRC cells, the PPARγ agonist rosiglitazone was observed to exert a protective effect in healthy intestinal tissues exposed to radiation, improve tissue structure, reduce intestinal apoptosis, and block inflammatory signaling cascades. These findings suggest that PPARγ agonists may effectively prevent and treat radiation-induced adverse conditions [99]. In addition, IFC-305, an adenosine derivative, inhibits methylation of the PPARγ promoter and up-regulates PPARγ expression, thereby reducing the risk of radiation-induced intestinal toxicity in colon cancer treatment [100]. It is well known that the Wnt/β-catenin signaling pathway plays an essential role in the development of CRC. β-catenin is a critical molecule in CRC [101]. Given that a potential crosstalk mechanism between PPARγ and Wnt/β-catenin regulates CRC progression, this offers a promising avenue for the development of novel PPARγ agonists that inhibit the Wnt/β-catenin signaling pathway [102]. Seetha and his colleagues found that the combination of indomethacin and juglone reduces the expression of inflammatory cytokines through the Wnt, Notch, and PPARγ pathways, thereby inducing apoptosis in colon cancer cells [103].
Non-coding RNAs (ncRNAs), which comprise the vast majority of the human genome, are a group of non-protein-coding RNAs [104, 105]. NcRNAs include microRNAs (miRNAs), long ncRNAs (lncRNAs), circular RNAs (circRNAs), and PIWI-interacting RNAs (piRNAs) [104,105,106]. Numerous studies have shown a potential association between ncRNAs and cancer development. NcRNAs modulate cancer by regulating gene expression and complex biological processes [105]. For example, circRNAs have been shown to regulate the progression of lung cancer, hepatocellular carcinoma, and glioma through the Wnt/β-catenin and PI3K/AKT pathways [104, 105, 107, 108]. MiRNAs can inhibit the PTEN/PI3K/Akt pathway, promoting growth in multiple myeloma (MM) [109]. Targeted miRNA-based therapies offer insights into potential treatments for glioma [110]. Of interest, a well-designed study by Zhang et al. using CRC cells showed that PPARγ is regulated by the LncRNA TINCR/microRNA-107/CD36 axis and that this pathway regulates tumor proliferation or apoptosis [111]. Surprisingly, a recent in vitro experiment concluded, contrary to the above, that inhibition of the PPARγ signaling pathway significantly inhibited the growth and accelerated the apoptosis of CRC cells [112]. A significant article summarizes the mechanisms through which pro-inflammatory cytokines, including the IL-1 family, IL-6, and TNF-α, exert pro-tumorigenic or tumor-suppressive effects during tumorigenesis. The levels of these cytokines within the TME may be correlated with cancer development [67]. A well-established example exemplifies that the PPARγ agonist 15-d-PGJ2 and troglitazone block the IL-6 signaling pathway through the inactivation of STAT3, which induces attenuated proliferation and increased apoptosis in MM cells [113].
Invasion and metastasis
Previous reports have shown that the PPARγ agonist troglitazone inhibits colon cancer metastasis by inhibiting matrix metalloproteinase-7 (MMP-7) synthesis and the adhesion of extracellular matrix (ECM) proteins [42, 114]. Twenty years ago, as a result of the establishment of xenograft animal models, the PPARγ ligand thiazolidinedione was shown to have the ability to inhibit the growth and metastasis of colon cancer cells by promoting differentiation effects. These effects are strongly linked to E-cadherin, β-catenin, and Drg-1 [115]. Nevertheless, later, Papi et al. proposed that the combination of 6-OH-11-O-hydroxyphenantrene (IIF) with PPARγ ligands, including ciglitazone and pioglitazone, inhibited the proliferation and invasion of colon cancer cell lines [116]. A recent study demonstrated that the pro-carcinogenic effects induced by IL-33, a product of Group 2 innate lymphoid cells (ILC2s), are driven by PPARγ. This pro-carcinogenic effect is characterized by increased CRC’s invasive and metastatic capacity without any impact on cell proliferation. By contrast, inhibition of PPARγ interferes with this effect [117]. Furthermore, Takano and colleagues found that the PPARγ agonist pioglitazone down-regulated COX-2 and cyclin D1 and inhibited colon cancer proliferation and liver metastasis [118]. Apart from this, recent studies have shown that the utilization of pioglitazone does not prevent ischemia/reperfusion injury (IRI)-induced liver metastasis in a mouse colon cancer model of IRI [119]. At present, PPARγ exhibits a paradoxical role in metastasis. Given the lack of inadequate research efforts in this area, it is impossible to hypothesize the exact role of PPARγ in CRC metastasis.
Tumor metabolism
The Wnt/β-catenin pathway negatively regulates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2) via PPARγ signaling. Subsequently, this results in an increase in glycolysis and the regulation of enterocyte differentiation [120]. Alternatively, the biosynthetic pathway of unsaturated fatty acids has been reported to contribute to the elevated risk of CRC observed in obese populations. Linoleic acid can inhibit this pathway, and PPARγ, which acts as a receptor for linoleic acid, plays an essential role in this process [121]. Further evidence substantiates the assertion that the upregulation of PPARγ by conjugated linoleic acid impedes colon cancer cell proliferation and stimulates apoptosis via interfering with glucose metabolic pathways and NAD+ levels [122]. In addition, PPARγ plays a pivotal role in combating oxidative stress. Zhang et al. found that the PPARγ agonist ELB00824 inhibited oxaliplatin-induced side effects—pain caused by oxidative stress and neuronal hypersensitivity. Nevertheless, it did not reduce the antitumor activity of oxaliplatin in human CRC cells in vitro [123]. We found that using the Xiao-Jian-Zhong formula in a vitro DSS-induced colitis mouse model promotes PPARγ expression by inhibiting pyroptosis and reactive oxygen species (ROS) levels. This protects intestinal mucosal integrity and prevents colitis-associated CRC [124]. Figure 3 provides a brief overview of the role of PPARγ in CRC.
Future and challenges
The mechanisms and functions of PPARs in cancer have been extensively studied. The results of this study illustrate the potential function and influence of PPAR in CRC. These findings demonstrate the potential impact of PPARs on CRC. Still, some questions need to be considered and resolved. First, the question of how PPAR affects the growth and progression of CRC through the TME is not well understood. Second, despite a large amount of preclinical evidence, there have been no reports on the widespread and effective use of PPAR modulators in the clinic. This may be attributed to the instability of PPARs in different cell and tissue models. This makes their drug development and application a colossal challenge. Developing new PPAR agonists or antagonists and exploring their potential role in the clinical setting are necessary. Apart from that, is there a strong relationship between the levels of PPARs and disease progression, and can we predict prognosis and disease status based on PPAR expression? Finally, as mentioned earlier, combining PPARs and other drugs and therapies provides a valuable tool for treating CRC. Therefore, more studies and clinical trials are needed to evaluate the impact of combining PPARs with other therapies, such as immunotherapy, chemotherapy, and radiotherapy, on CRC patients’ clinical efficacy and safety.
Conclusion
As one of the world’s deadliest malignant tumors, the exact pathogenesis of CRC remains unclear. Due to the non-specificity of early symptoms, patients are often diagnosed in advanced stages, which places a significant burden on their psychological state and physical well-being. In recent years, PPAR, a nuclear transcription factor, has been the subject of considerable research interest among the scientific community. This review summarizes the literature on PPAR and discusses the mechanisms involved in three PPARs in CRC. In the context of CRC, there is potential for designing endogenous or exogenous PPAR ligands as targeting agents. This offers new insights into the relevance of molecular targeting for cancer therapy. Therefore, future research must aim to more precisely elucidate the underlying mechanisms of CRC pathogenesis. The objective is to develop novel PPAR agonists and antagonists for more stable and practical application in clinical settings. Furthermore, the potential impact of combining PPAR modulators in patients with CRC merits investigation. PPAR modulators should be applied more widely in clinical practice, whether singly or combined. Undoubtedly, the role and targets of PPAR associated with CRC still need to be better understood, necessitating further research. We need to explore the association between CRC and PPAR more. This will facilitate the development of more effective therapeutic agents. It seems reasonable to posit that, as medical science advances, the pathophysiological mechanisms regulating PPAR in CRC will eventually be elucidated. Develop effective PPAR-targeted drugs. CRC will no longer be a global health concern.
References
Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal cancer: from risk factors to oncogenesis. Medicina. 2023;59:1646.
Sun Y, Zhang X, Hang D, Lau HC, Du J, Liu C, et al. Integrative plasma and fecal metabolomics identify functional metabolites in adenoma-colorectal cancer progression and as early diagnostic biomarkers. Cancer Cell. 2024;42:1386–400.e8.
Bian Y, Xu S, Gao Z, Ding J, Li C, Cui Z, et al. m(6)A modification of lncRNA ABHD11-AS1 promotes colorectal cancer progression and inhibits ferroptosis through TRIM21/IGF2BP2/ FOXM1 positive feedback loop. Cancer Lett. 2024;596:217004.
Ding XJ, Cai XM, Wang QQ, Liu N, Zhong WL, Xi XN, et al. Vitexicarpin suppresses malignant progression of colorectal cancer through affecting c-Myc ubiquitination by targeting IMPDH2. Phytomed Int J Phytother Phytopharmacol. 2024;132:155833.
Peng C, Tan Y, Yang P, Jin K, Zhang C, Peng W, et al. Circ-GALNT16 restrains colorectal cancer progression by enhancing the SUMOylation of hnRNPK. J Exp Clin Cancer Res. 2021;40:272.
Kim BJ, Hanna MH. Colorectal cancer in young adults. J Surg Oncol. 2023;127:1247–51.
Skalitzky MK, Zhou PP, Goffredo P, Guyton K, Sherman SK, Gribovskaja-Rupp I, et al. Characteristics and symptomatology of colorectal cancer in the young. Surgery. 2023;173:1137–43.
Celiberto F, Aloisio A, Girardi B, Pricci M, Iannone A, Russo F, et al. Fibres and colorectal cancer: clinical and molecular evidence. Int J Mol Sci. 2023;24:13501.
Chi T, Wang M, Wang X, Yang K, Xie F, Liao Z, et al. PPAR-γ modulators as current and potential cancer treatments. Front Oncol. 2021;11:737776.
Lee J, Kim SY. [Obesity and colorectal cancer]. Korean J Gastroenterol. 2023;82:63–72.
Papavassiliou AG, Delle Cave D. Novel Therapeutic Approaches for colorectal cancer treatment. Int J Mol Sci. 2024;25:2228.
Alipourgivi F, Motolani A, Qiu AY, Qiang W, Yang GY, Chen S, et al. Genetic alterations of NF-κB and Its regulators: a rich platform to advance colorectal cancer diagnosis and treatment. Int J Mol Sci. 2023;25:154.
Stalin A, Lin D, Josephine Princy J, Feng Y, Xiang H, Ignacimuthu S, et al. Computational analysis of single nucleotide polymorphisms (SNPs) in PPAR gamma associated with obesity, diabetes and cancer. J Biomol Struct Dyn. 2022;40:1843–57.
Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab Clin Exp. 2021;114:154338.
Xi Y, Zhang Y, Zhu S, Luo Y, Xu P, Huang Z. PPAR-mediated toxicology and applied pharmacology. Cells. 2020;9:352.
Sheng W, Wang Q, Qin H, Cao S, Wei Y, Weng J, et al. Osteoarthritis: role of peroxisome proliferator-activated receptors. Int J Mol Sci. 2023;24:13137.
Rapuano R, Ziccardi P, Cioffi V, Dallavalle S, Moricca S, Lupo A. Cladosporols A and B, two natural peroxisome proliferator-activated receptor gamma (PPARγ) agonists, inhibit adipogenesis in 3T3-L1 preadipocytes and cause a conditioned-culture-medium-dependent arrest of HT-29 cell proliferation. Biochim Biophys Acta Gen Subj. 2021;1865:129973.
Dhaini HR, Daher Z. Genetic polymorphisms of PPAR genes and human cancers: evidence for gene-environment interactions. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2019;37:146–79.
Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur J Med Chem. 2019;166:502–13.
Bahrambeigi S, Molaparast M, Sohrabi F, Seifi L, Faraji A, Fani S, et al. Targeting PPAR ligands as possible approaches for metabolic reprogramming of T cells in cancer immunotherapy. Immunol Lett. 2020;220:32–7.
Takada I, Makishima M. Peroxisome proliferator-activated receptor agonists and antagonists: a patent review (2014-present). Expert Opin Ther Pat. 2020;30:1–13.
Zhou D, Jin J, Liu Q, Shi J, Hou Y. PPARδ agonist enhances colitis-associated colorectal cancer. Eur J Pharmacol. 2019;842:248–54.
Yuvaraj S, Kumar BRP. Peroxisome proliferator-activated receptor-γ as a novel and promising target for treating cancer via regulation of inflammation: a brief review. Mini Rev Med Chem. 2022;22:3–14.
Yaghoubizadeh M, Pishkar L, Basati G. Aberrant expression of peroxisome proliferator-activated receptors in colorectal cancer and their association with cancer progression and prognosis. Gastrointest Tumors. 2020;7:11–20.
Wagner N, Wagner KD. The role of PPARs in disease. Cells. 2020;9:2367.
Zeng W, Yin X, Jiang Y, Jin L, Liang W. PPARα at the crossroad of metabolic-immune regulation in cancer. FEBS J. 2022;289:7726–39.
Font-Díaz J, Jiménez-Panizo A, Caelles C, Vivanco MD, Pérez P, Aranda A, et al. Nuclear receptors: lipid and hormone sensors with essential roles in the control of cancer development. Semin Cancer Biol. 2021;73:58–75.
Modica S, Gofflot F, Murzilli S, D’Orazio A, Salvatore L, Pellegrini F, et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 2010;138:636–48. 48.e1-12
De Lellis L, Cimini A, Veschi S, Benedetti E, Amoroso R, Cama A, et al. The anticancer potential of peroxisome proliferator-activated receptor antagonists. ChemMedChem. 2018;13:209–19.
De Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403.
Naser R, Fakhoury I, El-Fouani A, Abi-Habib R, El-Sibai M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy (Review). Int J Oncol. 2023;62:23.
Ballav S, Biswas B, Sahu VK, Ranjan A, Basu S. PPAR-γ partial agonists in disease-fate decision with special reference to cancer. Cells. 2022;11:3215.
Hanahan D, Monje M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell. 2023;41:573–80.
Luo Y, Xie C, Brocker CN, Fan J, Wu X, Feng L, et al. Intestinal PPARα protects against colon carcinogenesis via regulation of methyltransferases DNMT1 and PRMT6. Gastroenterology. 2019;157:744–59.e4.
Kong R, Wang N, Han W, Bao W, Lu J. Fenofibrate exerts antitumor effects in colon cancer via regulation of DNMT1 and CDKN2A. PPAR Res. 2021;2021:6663782.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8(+) T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res. 2018;6:1375–87.
Ammazzalorso A, De Lellis L, Florio R, Laghezza A, De Filippis B, Fantacuzzi M, et al. Synthesis of novel benzothiazole amides: evaluation of PPAR activity and anti-proliferative effects in paraganglioma, pancreatic and colorectal cancer cell lines. Bioorg Med Chem Lett. 2019;29:2302–6.
Siblini Y, Namour F, Oussalah A, Guéant JL, Chéry C. Stemness of normal and cancer cells: the influence of methionine needs and SIRT1/PGC-1α/PPAR-α players. Cells. 2022;11.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8.
Grau R, Punzón C, Fresno M, Iñiguez MA. Peroxisome-proliferator-activated receptor alpha agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochem J. 2006;395:81–8.
Dana N, Javanmard SH, Rafiee L. Role of peroxisome proliferator-activated receptor alpha and gamma in antiangiogenic effect of pomegranate peel extract. Iran J Basic Med Sci. 2016;19:106–10.
Wagner N, Wagner KD. Peroxisome proliferator-activated receptors and the hallmarks of cancer. Cells. 2022;11:2432.
Wagner N, Wagner KD. PPARs and angiogenesis-implications in pathology. Int J Mol Sci. 2020;21:5723.
Martinasso G, Oraldi M, Trombetta A, Maggiora M, Bertetto O, Canuto RA, et al. Involvement of PPARs in cell proliferation and apoptosis in human colon cancer specimens and in normal and cancer cell lines. PPAR Res. 2007;2007:93416.
Gao J, Liu Q, Xu Y, Gong X, Zhang R, Zhou C, et al. PPARα induces cell apoptosis by destructing Bcl2. Oncotarget. 2015;6:44635–42.
Li P, Zhang HY, Gao JZ, Du WQ, Tang D, Wang W, et al. Mesenchymal stem cells-derived extracellular vesicles containing miR-378a-3p inhibit the occurrence of inflammatory bowel disease by targeting GATA2. J Cell Mol Med. 2022;26:3133–46.
Gros B, Kaplan GG. Ulcerative colitis in adults: a review. JAMA. 2023;330:951–65.
Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55.
Chen W, Zou J, Shi X, Huang H. Downregulation of CPT1A exerts a protective effect in dextran sulfate sodium-induced ulcerative colitis partially by inhibiting PPARα signaling pathway. Drug Dev Res. 2022;83:1408–18.
Romano B, Pagano E, Iannotti FA, Piscitelli F, Brancaleone V, Lucariello G, et al. N-Acylethanolamine acid amidase (NAAA) is dysregulated in colorectal cancer patients and its inhibition reduces experimental cancer growth. Br J Pharmacol. 2022;179:1679–94.
Leng J, Li H, Niu Y, Chen K, Yuan X, Chen H, et al. Low-dose mono(2-ethylhexyl) phthalate promotes ovarian cancer development through PPARα-dependent PI3K/Akt/NF-κB pathway. Sci Total Environ. 2021;790:147990.
Dutta A, Sharma-Walia N. Curbing lipids: impacts ON cancer and viral infection. Int J Mol Sci. 2019;20:644.
Gou Q, Dong C, Jin J, Liu Q, Lu W, Shi J, et al. PPARα agonist alleviates tumor growth and chemo-resistance associated with the inhibition of glucose metabolic pathway. Eur J Pharmacol. 2019;863:172664.
Chen L, Jiao T, Liu W, Luo Y, Wang J, Guo X, et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell. 2022;29:1366–81.e9.
Rolver MG, Holland LKK, Ponniah M, Prasad NS, Yao J, Schnipper J, et al. Chronic acidosis rewires cancer cell metabolism through PPARα signaling. Int J Cancer. 2023;152:1668–84.
Yin X, Zeng W, Wu B, Wang L, Wang Z, Tian H, et al. PPARα inhibition overcomes tumor-derived exosomal lipid-induced dendritic cell dysfunction. Cell Rep. 2020;33:108278.
Wang D, Fu L, Wei J, Xiong Y, Dubois RN. PPARδ mediates the effect of dietary fat in promoting colorectal cancer metastasis. Cancer Res. 2019;79:4480–90.
Zuo X, Peng Z, Moussalli MJ, Morris JS, Broaddus RR, Fischer SM, et al. Targeted genetic disruption of peroxisome proliferator-activated receptor-delta and colonic tumorigenesis. J Natl Cancer Inst. 2009;101:762–7.
Zuo X, Xu M, Yu J, Wu Y, Moussalli MJ, Manyam GC, et al. Potentiation of colon cancer susceptibility in mice by colonic epithelial PPAR-δ/β overexpression. J Natl Cancer Inst. 2014;106:dju052.
Mao F, Xu M, Zuo X, Yu J, Xu W, Moussalli MJ, et al. 15-Lipoxygenase-1 suppression of colitis-associated colon cancer through inhibition of the IL-6/STAT3 signaling pathway. FASEB J. 2015;29:2359–70.
You M, Yuan S, Shi J, Hou Y. PPARδ signaling regulates colorectal cancer. Curr Pharm Des. 2015;21:2956–9.
Tan NS, Vázquez-Carrera M, Montagner A, Sng MK, Guillou H, Wahli W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Prog Lipid Res. 2016;64:98–122.
Wang D, Fu L, Ning W, Guo L, Sun X, Dey SK, et al. Peroxisome proliferator-activated receptor δ promotes colonic inflammation and tumor growth. Proc Natl Acad Sci USA. 2014;111:7084–9.
Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-delta attenuates colon carcinogenesis. Nat Med. 2004;10:481–3.
Rosales-Reynoso MA, Wence-Chavez LI, Arredondo-Valdez AR, Dumois-Petersen S, Barros-Núñez P, Gallegos-Arreola MP, et al. Protective role of +294 T/C (rs2016520) polymorphism of PPARD in Mexican patients with colorectal cancer. Genet Mol Res. 2017;16:gmr16019324.
Meissner M, Hrgovic I, Doll M, Naidenow J, Reichenbach G, Hailemariam-Jahn T, et al. Peroxisome proliferator-activated receptor {delta} activators induce IL-8 expression in nonstimulated endothelial cells in a transcriptional and posttranscriptional manner. J Biol Chem. 2010;285:33797–804.
Tahmasebi S, Alimohammadi M, Khorasani S, Rezaei N. Pro-tumorigenic and anti-tumorigenic roles of pro-inflammatory cytokines in cancer. In: Rezaei N, editor. Handbook of cancer and immunology. Cham: Springer International Publishing; 2022. p. 1–25.
Zuo X, Xu W, Xu M, Tian R, Moussalli MJ, Mao F, et al. Metastasis regulation by PPARD expression in cancer cells. JCI Insight. 2017;2:e91419.
Piqueras L, Reynolds AR, Hodivala-Dilke KM, Alfranca A, Redondo JM, Hatae T, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–9.
Wagner KD, Du S, Martin L, Leccia N, Michiels JF, Wagner N. Vascular PPARβ/δ promotes tumor angiogenesis and progression. Cells. 2019;8:1623.
Li Y, Cao R, Gu T, Cao C, Chen T, Guan Y, et al. PPARβ/δ augments IL-1β-induced COX-2 expression and PGE2 biosynthesis in human mesangial cells via the activation of SIRT1. Metabolites. 2022;12:595.
Liu Y, Colby JK, Zuo X, Jaoude J, Wei D, Shureiqi I. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci. 2018;19:3339.
Yoshinaga M, Kitamura Y, Chaen T, Yamashita S, Tsuruta S, Hisano T, et al. The simultaneous expression of peroxisome proliferator-activated receptor delta and cyclooxygenase-2 may enhance angiogenesis and tumor venous invasion in tissues of colorectal cancers. Dig Dis Sci. 2009;54:1108–14.
He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–45.
Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, Dubois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med. 2004;10:245–7.
Liou JY, Ghelani D, Yeh S, Wu KK. Nonsteroidal anti-inflammatory drugs induce colorectal cancer cell apoptosis by suppressing 14-3-3epsilon. Cancer Res. 2007;67:3185–91.
Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, et al. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:9968–73.
Wang D, Ning W, Xie D, Guo L, Dubois RN. Peroxisome proliferator-activated receptor δ confers resistance to peroxisome proliferator-activated receptor γ-induced apoptosis in colorectal cancer cells. Oncogene. 2012;31:1013–23.
Ding J, Gou Q, Jia X, Liu Q, Jin J, Shi J, et al. AMPK phosphorylates PPARδ to mediate its stabilization, inhibit glucose and glutamine uptake and colon tumor growth. J Biol Chem. 2021;297:100954.
Hao YH, Lafita-Navarro MC, Zacharias L, Borenstein-Auerbach N, Kim M, Barnes S, et al. Induction of LEF1 by MYC activates the WNT pathway and maintains cell proliferation. Cell Commun Signal. 2019;17:129.
Tan EHP, Sng MK, How ISB, Chan JSK, Chen J, Tan CK, et al. ROS release by PPARβ/δ-null fibroblasts reduces tumor load through epithelial antioxidant response. Oncogene. 2018;37:2067–78.
Mana MD, Hussey AM, Tzouanas CN, Imada S, Barrera Millan Y, Bahceci D, et al. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 2021;35:109212.
Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53–8.
Zhang W, Xu Y, Xu Q, Shi H, Shi J, Hou Y. PPARδ promotes tumor progression via activation of Glut1 and SLC1-A5 transcription. Carcinogenesis. 2017;38:748–55.
Ding J, Gou Q, Jin J, Shi J, Liu Q, Hou Y. Metformin inhibits PPARδ agonist-mediated tumor growth by reducing Glut1 and SLC1A5 expressions of cancer cells. Eur J Pharmacol. 2019;857:172425.
Gou Q, Zhang W, Xu Y, Jin J, Liu Q, Hou Y, et al. EGFR/PPARδ/HSP90 pathway mediates cancer cell metabolism and chemoresistance. J Cell Biochem. 2021;122:394–402.
Jeong E, Koo JE, Yeon SH, Kwak MK, Hwang DH, Lee JY. PPARδ deficiency disrupts hypoxia-mediated tumorigenic potential of colon cancer cells. Mol Carcinog. 2014;53:926–37.
Dubois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998;19:49–53.
Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, et al. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4:1058–61.
Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, et al. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053–7.
Vallée A, Lecarpentier Y. Crosstalk between peroxisome proliferator-activated receptor gamma and the canonical WNT/β-catenin pathway in chronic inflammation and oxidative stress during carcinogenesis. Front Immunol. 2018;9:745.
Cui Y, Zhang C, Wang Y, Ma S, Cao W, Guan F. HOXC11 functions as a novel oncogene in human colon adenocarcinoma and kidney renal clear cell carcinoma. Life Sci. 2020;243:117230.
Peng C, Xu Y, Wu J, Wu D, Zhou L, Xia X. TME-related biomimetic strategies against cancer. Int J Nanomed. 2024;19:109–35.
Tahmasebi S, Elahi R, Esmaeilzadeh A. Solid tumors challenges and new insights of CAR T cell engineering. Stem Cell Rev Rep. 2019;15:619–36.
Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12:81.
Huang G, Yin L, Lan J, Tong R, Li M, Na F, et al. Synergy between peroxisome proliferator-activated receptor γ agonist and radiotherapy in cancer. Cancer Sci. 2018;109:2243–55.
Biswas P, Swaroop S, Dutta N, Arya A, Ghosh S, Dhabal S, et al. IL-13 and the hydroperoxy fatty acid 13(S)HpODE play crucial role in inducing an apoptotic pathway in cancer cells involving MAO-A/ROS/p53/p21 signaling axis. Free Radic Biol Med. 2023;195:309–28.
Sabatino L, Ziccardi P, Cerchia C, Muccillo L, Piemontese L, Loiodice F, et al. Chiral phenoxyacetic acid analogues inhibit colon cancer cell proliferation acting as PPARγ partial agonists. Sci Rep. 2019;9:5434.
Mangoni M, Sottili M, Gerini C, Desideri I, Bastida C, Pallotta S, et al. A PPAR-gamma agonist protects from radiation-induced intestinal toxicity. U Eur Gastroenterol J. 2017;5:218–26.
Lian B, Ren Y, Zhang H, Lin T, Wang Y. An adenosine derivative (IFC-305) reduced the risk of radiation-induced intestinal toxicity in the treatment of colon cancer by suppressing the methylation of PPAR-r promoter. Biomed Pharmacother. 2019;118:109202.
Villa ALP, Parra RS, Feitosa MR, Camargo HP, Machado VF, Tirapelli D, et al. PPARG expression in colorectal cancer and its association with staging and clinical evolution. Acta Cir Bras. 2020;35:e202000708.
Sabatino L, Pancione M, Votino C, Colangelo T, Lupo A, Novellino E, et al. Emerging role of the β-catenin-PPARγ axis in the pathogenesis of colorectal cancer. World J Gastroenterol. 2014;20:7137–51.
Seetha A, Devaraj H, Sudhandiran G. Indomethacin and juglone inhibit inflammatory molecules to induce apoptosis in colon cancer cells. J Biochem Mol Toxicol. 2020;34:e22433.
Alimohammadi M, Gholinezhad Y, Mousavi V, Kahkesh S, Rezaee M, Yaghoobi A, et al. Circular RNAs: novel actors of Wnt signaling pathway in lung cancer progression. EXCLI J. 2023;22:645–69.
Mafi A, Rismanchi H, Malek Mohammadi M, Hedayati N, Ghorbanhosseini SS, Hosseini SA, et al. A spotlight on the interplay between Wnt/β-catenin signaling and circular RNAs in hepatocellular carcinoma progression. Front Oncol. 2023;13:1224138.
Saw PE, Xu X, Chen J, Song EW. Non-coding RNAs: the new central dogma of cancer biology. Sci China Life Sci. 2021;64:22–50.
Kahkesh S, Khoshnazar SM, Gholinezhad Y, Esmailzadeh S, Hosseini SA, Alimohammadi M, et al. The potential role of circular RNAs -regulated PI3K signaling in non-small cell lung cancer: molecular insights and clinical perspective. Pathol Res Pract. 2024;257:155316.
Mafi A, Khoshnazar SM, Shahpar A, Nabavi N, Hedayati N, Alimohammadi M, et al. Mechanistic insights into circRNA-mediated regulation of PI3K signaling pathway in glioma progression. Pathol Res Pract. 2024;260:155442.
Alimohammadi M, Rahimzadeh P, Khorrami R, Bonyadi M, Daneshi S, Nabavi N, et al. A comprehensive review of the PTEN/PI3K/Akt axis in multiple myeloma: from molecular interactions to potential therapeutic targets. Pathol Res Pract. 2024;260:155401.
Mafi A, Mannani R, Khalilollah S, Hedayati N, Salami R, Rezaee M, et al. The significant role of microRNAs in gliomas angiogenesis: a particular focus on molecular mechanisms and opportunities for clinical application. Cell Mol Neurobiol. 2023;43:3277–99.
Zhang X, Yao J, Shi H, Gao B, Zhang L. LncRNA TINCR/microRNA-107/CD36 regulates cell proliferation and apoptosis in colorectal cancer via PPAR signaling pathway based on bioinformatics analysis. Biol Chem. 2019;400:663–75.
Wang R, Li J, Zhou X, Mao Y, Wang W, Gao S, et al. Single-cell genomic and transcriptomic landscapes of primary and metastatic colorectal cancer tumors. Genome Med. 2022;14:93.
Wang LH, Yang XY, Zhang X, Huang J, Hou J, Li J, et al. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity. 2004;20:205–18.
Sunami E, Tsuno NH, Kitayama J, Saito S, Osada T, Yamaguchi H, et al. Decreased synthesis of matrix metalloproteinase-7 and adhesion to the extracellular matrix proteins of human colon cancer cells treated with troglitazone. Surg Today. 2002;32:343–50.
Yoshizumi T, Ohta T, Ninomiya I, Terada I, Fushida S, Fujimura T, et al. Thiazolidinedione, a peroxisome proliferator-activated receptor-gamma ligand, inhibits growth and metastasis of HT-29 human colon cancer cells through differentiation-promoting effects. Int J Oncol. 2004;25:631–9.
Papi A, Rocchi P, Ferreri AM, Orlandi M. RXRgamma and PPARgamma ligands in combination to inhibit proliferation and invasiveness in colon cancer cells. Cancer Lett. 2010;297:65–74.
Ercolano G, Gomez-Cadena A, Dumauthioz N, Vanoni G, Kreutzfeldt M, Wyss T, et al. PPARɣ drives IL-33-dependent ILC2 pro-tumoral functions. Nat Commun. 2021;12:2538.
Takano S, Kubota T, Nishibori H, Hasegawa H, Ishii Y, Nitori N, et al. Pioglitazone, a ligand for peroxisome proliferator-activated receptor-gamma acts as an inhibitor of colon cancer liver metastasis. Anticancer Res. 2008;28:3593–9.
Tashiro Y, Nishino H, Higuchi T, Sugisawa N, Fukuda Y, Yamamoto J, et al. Ischemia reperfusion-induced metastasis is resistant to PPARγ agonist pioglitazone in a murine model of colon cancer. Sci Rep. 2020;10:18565.
Kim JT, Li C, Weiss HL, Zhou Y, Liu C, Wang Q, et al. Regulation of ketogenic enzyme HMGCS2 by Wnt/β-catenin/PPARγ pathway in intestinal cells. Cells. 2019;8:1106.
Mokhtari K, Mahdevar M, Hajipour M, Esmaeili M, Peymani M, Mirzaei S, et al. Involvement of unsaturated fatty acid biosynthesis in CRC progression based on in vitro and in silico studies. Biomed Pharmacother. 2022;153:113338.
Dubey V, Mishra AK, Ghosh AR. Appraisal of the possible role of PPARγ upregulation by CLA of probiotic Pediococcus pentosaceus GS4 in colon cancer mitigation. PPAR Res. 2023;2023:9458308.
Zhang M, Hu M, Alles SRA, Montera MA, Adams I, Santi MD, et al. Peroxisome proliferator-activated receptor gamma agonist ELB00824 suppresses oxaliplatin-induced pain, neuronal hypersensitivity, and oxidative stress. Neuropharmacology. 2022;218:109233.
Yu W, Liang Z, Li Q, Liu Y, Liu X, Jiang L, et al. The pharmacological validation of the Xiao-Jian-Zhong formula against ulcerative colitis by network pharmacology integrated with metabolomics. J Ethnopharmacol. 2022;298:115647.
Wagner N, Wagner KD. PPAR beta/delta and the hallmarks of cancer. Cells. 2020;9:1133.
Decara J, Rivera P, López-Gambero AJ, Serrano A, Pavón FJ, Baixeras E, et al. Peroxisome proliferator-activated receptors: experimental targeting for the treatment of inflammatory bowel diseases. Front Pharmacol. 2020;11:730.
Funding
Science and Technology Innovation Fund Project of Dalian (2023JJ13SN050); Natural Science Foundation of Liaoning Province (2024-MS-281).
Author information
Authors and Affiliations
Contributions
CW, TL, and BJ wrote the main manuscript text and CW prepared figures and table. ZF and YL revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Yes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Lv, T., Jin, B. et al. Regulatory role of PPAR in colorectal cancer. Cell Death Discov. 11, 28 (2025). https://doi.org/10.1038/s41420-025-02313-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-025-02313-2