Abstract
Ischemia-reperfusion injury (IRI) is a critical condition that poses a significant threat to patient safety. The production of lactate increases during the process of IRI, and lactate serves as a crucial indicator for assessing the severity of such injury. Lactylation, a newly discovered post-translational modification in 2019, is induced by lactic acid and predominantly occurs on lysine residues of histone or nonhistone proteins. Extensive studies have demonstrated the pivotal role of lactylation in the pathogenesis and progression of various diseases, including melanoma, myocardial infarction, hepatocellular carcinoma, Alzheimer’s disease, and nonalcoholic fatty liver disease. Additionally, a marked correlation between lactylation and inflammation has been observed. This article provides a comprehensive review of the mechanism underlying lactylation in IRI to establish a theoretical foundation for better understanding the interplay between lactylation and IRI.

Similar content being viewed by others
Facts
-
Lactylation can occur on lysine residues of both histones and nonhistone proteins.
-
Lactylation directly regulate gene expression and protein function.
-
Lactylation plays a significant role in ischemia-reperfusion injury.
Open questions
-
In the context of ischemia-reperfusion injury, which exerts a greater impact, acetylation or lactylation?
-
How do different post-translational modification mechanisms influence each other?
-
Is there a crosstalk between lactylation in histones and nonhistone proteins?
-
How does ischemia-reperfusion injury coordinate its effects across different organs, and through what pathways?
Introduction
Life’s sustenance hinges on a crucial equilibrium between oxygen supply and demand at the cellular level, a balance maintained through adequate blood flow perfusion [1]. Ischemia-reperfusion injury (IRI), a condition of paramount clinical significance, poses a substantial threat to patient safety [2]. Ischemia is characterized by reduced blood supply due to arterial obstruction, leading to tissue damage from inadequate nutrient and oxygen delivery. The subsequent process of reperfusion often aggravates tissue damage and promotes necrosis [3, 4]. A plethora of studies have indicated that various clinical scenarios, including sepsis, organ transplantation, shock, myocardial infarction, cerebral ischemia, and stroke, can precipitate IRI [5,6,7,8,9,10]. The effective management of systemic inflammatory response syndrome and multiple organ dysfunction syndrome, as consequences of IRI, remains a focal point in both clinical and basic research [11, 12]. Furthermore, the process of IRI involves the generation and accumulation of lactate, attributed to anaerobic glycolysis during ischemia and impaired cellular function during reperfusion [5,6,7,8,9,10,11,12]. Lactate, therefore, is an essential biomarker for gauging the severity of diseases associated with IRI. For instance, in intestinal IRI, D-lactate has been established as one of its specific diagnostic biomarkers [13].
Since its identification in 1780, lactate has been acknowledged as a metabolic byproduct generated under hypoxic conditions, known for its various adverse effects [14]. However, recent advancements, such as the lactate shuttle hypothesis, which posits lactate’s active involvement in oxidation, gluconeogenic substrates, and cellular signaling, have shifted the focus toward its broader roles beyond mere energy metabolism [15]. In 2019, the research team led by Zhao Yingming introduced the concept of lactylation, a groundbreaking revelation that highlighted lactate’s significant role in modulating histone lysine residue modifications [16]. This process offers a stark contrast to transcriptional regulation, as post-translational modifications (PTMs) like acetylation, succinylation, and malonylation can rapidly and precisely modulate protein function [17, 18]. As a novel PTM, lactylation has been extensively studied, revealing its pivotal involvement in the development and progression of various diseases, including melanoma, myocardial infarction, hepatocellular carcinoma, Alzheimer’s disease, and nonalcoholic fatty liver disease [18,19,20,21,22,23,24]. Additionally, a marked correlation between lactylation and inflammation has been observed [25,26,27,28].
Given the critical role of inflammation in IRI and the pronounced increase in lactate production during such injury, coupled with its diagnostic relevance in gauging disease severity, we hypothesize a significant interplay between lactylation and IRI. This review aims to provide a comprehensive analysis of the intricate relationship between lactylation and IRI, offering a solid theoretical foundation and potential research directions for IRI treatment and research.
Current status of IRI treatment: diverse research directions, yet effective intervention strategies remain insufficient
IRI refers to the phenomenon where tissue or organ damage is unexpectedly exacerbated once the blood supply is restored after a period of ischemia. Current IRI research extensively focuses on various aspects, including oxidative stress, calcium overload, mitochondrial dysfunction, and inflammatory responses [3, 29]. To address this complex issue, researchers have explored diverse therapeutic strategies, including but not limited to remote ischemic preconditioning, remote ischemic postconditioning, and pharmacological interventions such as antioxidants, anti-inflammatory drugs, calcium channel blockers, as well as hypothermia treatment [30,31,32]. While these research directions and treatment methods have shown some efficacy in improving IRI outcomes, a significant gap still exists between basic research and practical clinical applications. A notable challenge is that many strategies demonstrating promising results in animal models show diminished efficacy when translated to human clinical trials. Moreover, determining the precise timing for treatment, especially the critical time point for reperfusion postconditioning, remains a major challenge that continues to perplex researchers [33].
Mitochondrial dysfunction plays a central role in the IRI process. During the ischemic phase, the rapid depletion of ATP leads to a significant decrease in mitochondrial membrane potential, which in turn triggers electron transport chain dysfunction. When blood flow is restored, the influx of oxygen causes electrons to leak from the electron transport chain and combine with oxygen molecules, producing large amounts of reactive oxygen species (ROS). Simultaneously, the opening of the mitochondrial permeability transition pore induces mitochondrial swelling and rupture, releasing apoptotic factors and ultimately leading to cell death [34, 35]. Furthermore, under hypoxic conditions, glycolysis is significantly enhanced, leading to lactate accumulation. There is a complex interplay between ROS and glycolysis, with ROS regulating the activity of key glycolytic enzymes through various pathways, further promoting lactate production [28, 36]. As an important substrate for lactylation, the increase in lactate concentration significantly enhances the level of lactylation and has a crucial impact on the final outcome of IRI.
PTMs play a vital role in biological systems as a mechanism for rapid and precise regulation of protein function. Lactylation, a recently discovered important form of PTM, plays a key role in various IRI-related diseases, such as myocardial infarction, septic shock, and cerebral IRI [20, 37, 38]. Given that lactylation has rapid, precise, and energy-efficient regulatory characteristics and is closely related to key mitochondrial damage in IRI, an in-depth study of the connection between lactylation and IRI will help us better understand the pathological mechanisms of IRI and potentially discover new therapeutic targets.
Lactate metabolism
Sources of lactate and its role as a signaling molecule
Lactate plays a pivotal role in energy metabolism regulation and signal transduction, acting as a key cellular fuel source, particularly during intense physical activity and infection, when an imbalance exists between oxygen supply and ATP demand [15]. Under conditions of limited cellular oxygen availability, such as hypoxia or anaerobic environments, mitochondrial oxidative phosphorylation is hindered, leading to suboptimal pyruvate utilization. As a result, cells predominantly rely on glycolysis for energy production, culminating in lactate formation. Lactate is primarily synthesized during glycolysis as a common byproduct of glucose metabolism. In anaerobic or hypoxic conditions, glucose is metabolized into two molecules of pyruvate within the cytoplasm [14]. However, the lack of sufficient oxygen for electron acceptance in the electron transport chain prevents pyruvate from entering the mitochondria for further oxidation. At this juncture, lactate dehydrogenase facilitates the conversion of pyruvate into lactate, simultaneously replenishing NAD+ levels, thereby enabling the continuation of glycolysis [14, 15] (Fig. 1).
Lactate production and metabolism within cells encompass several key steps: Initially, lactate is translocated across the cellular membrane via the MCT, moving from the extracellular environment into the cytoplasm. Lactate formation occurs either through glycolytic processes or from the breakdown of glutamine. Cellular lactate catabolism is divided into two primary pathways. The first involves the oxidation of lactate to pyruvate, which is then transported into the mitochondria for subsequent catabolism within the TCA cycle. The second pathway involves the conversion of lactate back to glucose via gluconeogenesis. α-KG α-ketoglutaric acid, MCT monocarboxylate transporter, PDH pyruvate dehydrogenase, TCA tricarboxylic acid. Created with Figdraw.
The elucidation of the Warburg effect has significantly advanced our comprehension of lactate metabolism [16]. This phenomenon demonstrates that, even in the presence of oxygen, tumor cells predominantly utilize glycolysis over mitochondrial oxidative phosphorylation for energy production [39]. The Warburg effect facilitates the rapid proliferation of tumor cells in an oxygen-rich milieu by primarily depending on glycolysis for energy. Moreover, it contributes to tumor growth by supplying necessary growth factors and aids in evading immune detection [16, 39].
Monocarboxylate transporters (MCTs) are a family of solute carrier proteins chiefly responsible for the movement of one-carbon metabolites, such as lactate and keto acids, between cells. In humans, the MCTs encompasses several members, with MCT1 and MCT4 being the most thoroughly researched [40]. These transporters operate via a proton-linked mechanism, allowing lactate molecules to cross the cell membrane in tandem with protons (H+). This process maintains a dynamic equilibrium, regulating the concentration gradients of lactate and protons. Typically, MCTs facilitate passive transport, enabling lactate to move in or out of cells based on the direction of the concentration gradient [15, 40]. These transporters are instrumental in maintaining the balance of lactate levels both inside and outside the cell, especially in hyperglycolytic conditions such as those found in tumor cells [40]. Investigating these transporters not only deepens our understanding of regulatory mechanisms in cellular metabolism but also presents potential therapeutic avenues for certain diseases.
Currently, lactate fulfills three key roles: it acts as the primary energy source, serves as a significant precursor for gluconeogenesis, and functions as signaling molecules with autocrine, paracrine, and endocrine-like actions, collectively referred to as “lactormone” [41]. For example, recent studies have demonstrated the multifaceted role of lactate in various physiological and pathological processes. Notably, lactate has been shown to induce hypoxic responses, while inhibition of intracellular lactate production correspondingly suppresses these responses [42]. Furthermore, mitochondrial antiviral signaling protein has been identified as a direct sensor of lactate, thus establishing a link between energy metabolism and innate immunity [43]. Intriguingly, the continuous accumulation of lactate promotes the remodeling of abnormal late-promoting complexes and overcomes the effects of antimitotic drugs through mitotic slippage mechanisms, thereby regulating cell cycle progression and proliferation [44]. These findings collectively underscore the critical role of lactate in diverse pathophysiological processes.
The synthesis of lactate is an adaptive biological response, allowing cells to sustain energy metabolism via distinct pathways under aerobic and hypoxic conditions. In an aerobic environment, the generation of lactate aids in maintaining the equilibrium between glycolysis and mitochondrial metabolism. Conversely, under hypoxic conditions, the production of lactate becomes essential to facilitate the continuous operation of glycolysis.
Lactate regulates immune cells and inflammatory responses
During inflammation, the heightened energy requirements of immune cells (such as macrophages and T cells) are predominantly met through an increase in the glycolytic process, leading to significant lactate production [45]. These metabolic shifts not only supply essential energy to immune cells but may also modulate the inflammatory response via lactate or its derivatives. Lactate functions not merely as a metabolite but also as a signaling molecule, influencing inflammation by regulating immune cell behavior [46]. Additionally, lactate accumulation contributes to the acidification of the local microenvironment, potentially impacting immune cell functions, including T-cell activity and cytokine production [45] (Fig. 2).
Lactate plays a significant role in cellular metabolism by inhibiting mitochondrial oxidative phosphorylation, thereby contributing to mitochondrial dysfunction. Moreover, lactate demonstrates the capacity to modulate the activation of immune cells such as T cells, B cells, NK cells, neutrophils, macrophages, and mast cells. This modulation leads to the secretion of specific cytokines, which exert a profound influence on the inflammatory response. Arg1 arginase-1, CXCL CXC motif chemokine, G-CSF granulocyte colony-stimulating factor, GPR81 G-protein-coupled receptor 81, IFN-γ interferon-γ, IFNs type I interferons, IL-17 interleukin-17, NKp46 lysis receptor 46 of natural killer cell, PD-1 programmed cell death protein 1, PD-L1 programmed cell death protein 1 ligand, Th1 type 1 helper T cells, Th17 type 17 helper T cells, Tregs regulatory T cells, VEGF vascular endothelial growth factor. Created with Figdraw.
Lactate’s role varies with the metabolic state and specific cell type under investigation, impacting various pathological processes. It influences signaling pathways through mechanisms such as PTMs, interaction with G-protein-coupled receptors, and activation of transcription factors like NF-κB and HIF1. These interactions regulate the expression of cytokines, chemokines, adhesion molecules, and enzymes integral to immune responses and metabolic processes [47]. Moreover, lactate is intimately associated with the activation states of various immune cells, including monocytes, natural killer cells, mast cells, T cells, tumor cells, fibroblasts, and macrophages. In response to harmful stimuli, lactate can modulate the differentiation processes of Th1, Th17, and regulatory T cells, which are essential for maintaining immune homeostasis [48]. These insights imply that targeting lactate regulation could be a promising therapeutic approach for treating inflammatory diseases.
Targeting lactate and related inflammatory responses to improve IRI prognosis
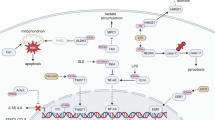
IRI is critical in the pathogenesis and advancement of many diseases. During ischemia, cellular ATP production through oxidative phosphorylation is compromised due to a restricted oxygen supply. This limitation forces cells to depend on anaerobic metabolism for lactate generation. Subsequently, lactate accumulation causes a decrease in intracellular pH, negatively impacting cellular functionality and potentially inducing cell death [41]. Prolonged ischemic conditions intensify this metabolic disturbance by further reducing ATP levels and intracellular pH, a result of sustained anaerobic metabolism and lactate accumulation. This leads to a dysfunction in ATP-dependent ion transport processes, culminating in increased intracellular and mitochondrial calcium levels (calcium overload), cellular swelling, rupture, and cell death via various pathways including necrosis, apoptosis, and autophagy. Despite reperfusion reestablishing oxygenation, it paradoxically exacerbates the injury by promoting reactive oxygen species production and encouraging pro-inflammatory neutrophils to infiltrate the ischemic tissues, thereby amplifying the ischemic damage [49] (Fig. 3).
In the context of ischemia-reperfusion injury, there is a reduction in intracellular ATP levels and pH, attributed to anaerobic metabolism and the accumulation of lactate. Consequently, there is a compromise in the ATP-dependent ion transport mechanism. This disruption precipitates intracellular and intra-mitochondrial calcium overload, leading to mitochondrial dysfunction and a subsequent upsurge in the production of inflammatory factors. Additionally, the release of the corresponding cytokines and chemokines induces immune cell infiltration. These biochemical alterations result in cellular swelling, rupture, and demise. Modes of cell death encompass necrosis, apoptosis, pyroptosis, autophagy, and other associated pathways. IL-1β interleukin-1β, ROS reactive oxygen species, TNF-α tumor necrosis factor-α. Created with Figdraw.
IRI is known to initiate both local and systemic inflammatory responses. During the ischemic phase, cells undergoing stress release a multitude of inflammatory mediators, including cytokines and chemokines. These mediators further stimulate immune cell activation during reperfusion [49]. In this phase, the resultant cellular injury and death precipitate the secretion of various inflammatory agents (e.g., tumor necrosis factor-α, interleukins) and chemoattractants, leading to significant tissue inflammation and damage [50]. This inflammatory cascade increases vascular permeability, resulting in the leakage of plasma components into adjacent tissues, which contributes to tissue swelling and further aggravates the damage [51]. Upon reestablishment of blood flow following prolonged ischemia, the local inflammatory response and reactive oxygen species production intensify, thereby exacerbating secondary tissue injury. The cellular damage caused by chronic IRI can culminate in various forms of cell death, including apoptosis, autophagy, necrosis, and necroptosis [3].
The management of lactate levels and inflammatory processes is crucial in the development and progression of IRI. Consequently, targeting these aspects not only diminishes the severity of IRI but also provides innovative strategies for treating related diseases. For instance, postconditioning with lactate-enriched blood has been shown to mitigate fatal human reperfusion injury, highlighting the potential of lactate-based interventions in improving IRI outcomes [52]. The integration of these insights into clinical practice promises to improve therapeutic outcomes and enhance patient quality of life.
Lactylation - a novel PTM with lactate as the substrate
PTMs profoundly affect almost all proteins, playing a vital role in preserving the functionality and diversity of the proteome. Recent research has unveiled that lactate can facilitate the lysine lactylation (Kla) of core histone proteins, thereby modulating gene expression (e.g., Arg1) and influencing the pathogenesis of various conditions, such as tumor formation [16, 21] (Fig. 4). Subsequent studies have extended these findings to nonhistone proteins, exemplified by modifications in high mobility group box-1 (HMGB1) [28]. Lactylation has emerged as a pivotal factor in a spectrum of diseases, encompassing inflammation, multiple cancers, cognitive disorders, sepsis, and more [21, 23, 28, 41].
Lactate transport from extracellular to intracellular compartments, coupled with its generation via glycolysis, facilitates the lactylation of lysine residues on both histone and nonhistone proteins. This modification occurs through either enzyme-dependent or enzyme-independent pathways. Such a process directly influences protein function and plays a crucial role in the regulation of gene expression. AARS mitochondrial alanyl-tRNA synthetase, Brg1 brahma-related gene 1, GLUT glucose transporter, HBO1 histone acetyltransferase binding to ORC-1, HDAC histone deacetylase, Kla lysine lactylation, LGSH lactyl-glutathione, MCT monocarboxylate transporter, P300 acetyltransferase p300, SIRT histone deacetylase sirtuin. Created with Figdraw.
Since its discovery in 2019, lactylation has rapidly emerged as a promising research direction [16]. Initial investigations primarily focused on histone modifications, particularly at key sites such as H3K9, H3K18, H4K5, H4K8, and H4K12. The pivotal role of these sites in the pathogenesis of related diseases has been extensively validated through diverse methodologies [16, 53, 54]. With the advent of advanced technologies such as deep sequencing, the scope of lactylation research has expanded to encompass nonhistone proteins. This includes various proteins such as HMGB1, METTL3, PKM2, and AK2, and spans a wide range of diseases including sepsis, liver IRI, colon cancer, skin wounds, and hepatocellular carcinoma [21, 28, 55, 56]. Current research trends indicate parallel development in both histone and nonhistone lactylation, with an increasing focus on the latter. A growing body of evidence highlights the crucial role of lactylation in various diseases, particularly in tumor-related and IRI-related conditions associated with elevated lactate levels [18,19,20,21,22].
Moreover, there is a close interplay between lactylation and inflammatory mediators. A study on spinal cord injury demonstrated that the introduction of lactate significantly augments lactylation levels in microglia, effectively inhibiting the expression of pro-inflammatory mediators such as IL-1β, iNOS, and TNF-α [57]. Corroborating these findings, another study on experimental autoimmune uveitis (EAU) revealed enhanced lactate modification of Yin Yang-1 (YY1) in retinal microglia. Notably, YY1 lactylation not only promoted microglial activation but also enhanced their proliferation and migration capabilities. Inhibition of lactylation significantly suppressed microglial activation in EAU, thereby attenuating the inflammatory response [58]. These findings collectively underscore the significant role of lactylation in regulating inflammatory mediators and its involvement in disease progression. However, there is currently a paucity of research investigating whether inflammatory mediators can, in turn, modulate lactylation levels. Further studies are warranted to elucidate the potential regulatory effects of inflammatory mediators on lactylation.
Overall, since its initial identification in 2019, research on protein lactylation has evolved through several distinct developmental phases. Initial investigations predominantly focused on histone modifications, particularly at H3K9 and H3K18 sites, establishing their fundamental roles in transcriptional regulation [16, 53, 54]. Subsequently, the research scope expanded significantly to encompass nonhistone proteins, unveiling complex cellular regulatory networks. Contemporary literature analysis has revealed three major trends in lactylation research: First, lactylation modifications exert predominant effects on proteins involved in metabolic regulation and inflammatory responses, as demonstrated by: PKM2’s regulatory role in macrophage metabolism [56], HMGB1’s critical function in inflammatory responses [28], AK2’s essential involvement in cellular energy homeostasis [21]; Second, substantial crosstalk exists between lactylation and other PTMs, particularly acetylation, as evidenced by: The dual regulatory functionality of HDACs [59, 60], The dynamic equilibrium between lactylation and acetylation modifications of macrophage HMGB1 [28]; Third, lactylation modifications exhibit distinct tissue-specific distribution patterns, with notably higher prevalence in metabolically active tissues, as revealed by comprehensive proteomic analyses demonstrating elevated occurrence in: liver kupffer cells [61], cardiac tissue [62] and tumor microenvironment [22]. Given lactate’s pivotal role in energy metabolism and signal transduction, systematic investigation of both histone and nonhistone lactylation modifications has become essential. These investigations are particularly crucial for enhancing our understanding of the molecular mechanisms underlying inflammatory and metabolic disease development.
Histone lactylation
Histones, alkaline proteins located within the chromatin of eukaryotic cells, form nucleosome structures with DNA and are integral to chromatin architecture [63]. Histones undergo various PTMs, such as acetylation, malonylation, and succinylation. These modifications can alter the spatial structure of proteins, thereby regulating a wide array of cellular physiological and biochemical processes [64]. The study by Zhang et al. [16] revealed that lactate induces histone lysine lactylation, influencing gene transcription in a dose-dependent manner. Furthermore, they identified the enzyme acetyltransferase p300(p300), a renowned histone acetyltransferase that is recruited by P53, as responsible for modifying histone Kla [16, 18]. In a significant advancement in 2022, Zhang et al. delineated the role of Class I histone deacetylases (HDAC1–3) and histone deacetylase sirtuins (SIRT1–3) as delactylases in vitro. This discovery bridged a critical gap in delactylation research, integrating the novel lactylation PTM into a cohesive biochemical process [18, 59].
Additionally, lactyl-CoA, identified in mammalian cells and tissues via liquid chromatography-mass spectrometry, acts as the substrate for enzymatic lactylation. Non-enzymatic lactylation also occurs, involving the transfer of the lactyl group by lactyl-glutathione. These two distinct mechanisms can independently or jointly contribute to lactylation [22]. The modification of lysine residues through lactylation and acetylation significantly impacts the levels of metabolites such as lactate, acetyl-CoA, and pyruvic acid, as well as their intricate interplay. These metabolic alterations have profound implications for subsequent cellular pathological and physiological processes and revealed the potential correlation between lactylation and acetylation, given their similar mechanisms involving writers, erasers, and readers [18, 60].
Currently, the roles of Kla sites on five histone proteins in various diseases have been elucidated, with particular emphasis on the K18 site of histone 3 (H3K18). This site has been the subject of intensive research in human, rat, and mouse models, as summarized in Table 1. Histone lactylation has been predominantly identified in tumor-associated diseases, including lung cancer, melanoma, breast cancer, prostate cancer, colon cancer, renal cell carcinoma, colorectal cancer, and bladder cancer. Tumors are known to undergo metabolic reprogramming, tumor-associated inflammation, evasion of immunosurveillance, and genome instability. These changes facilitate sustained proliferation, resistance to apoptosis, evasion of growth suppressors, replicative immortality, tumor angiogenesis, and invasion and metastasis [22]. The Warburg effect, characterized by aerobic glycolysis, is closely linked to the onset and progression of tumors and promotes lactate production. This underscores the interconnection between lactylation and tumor biology, as evidenced by extensive research [16, 22]. For example, histone lactylation has been shown to drive oncogenesis by enhancing m6A reader protein YTH N6-methyladenosine RNA-binding protein 2 (YTHDF2) expression in ocular melanoma [19].
Beyond its significant correlation with cancer, histone lactylation is also implicated in a range of diseases, including preeclampsia, endometriosis, septic shock, myocardial infarction, Alzheimer’s disease, and various intestinal disorders characterized by inflammation [20, 37, 65,66,67,68]. These associations underscore the role of lactate as an extracellular energy substrate in epigenetic regulation, particularly through histone lactylation. During ischemic episodes, where oxygen deprivation leads to anaerobic glycolysis, there is a notable increase in lactate production, resulting in enhanced lactylation. The effectiveness of therapeutic strategies targeting histone lactylation sites in mitigating IRI and facilitating the recovery of associated functions has been validated in several studies [20, 37, 38]. For example, recent studies have elucidated the pivotal role of histone lactylation in regulating the dual functions of monocyte-macrophages, specifically their anti-inflammatory and pro-angiogenic properties, in the context of myocardial infarction (MI). This process is mediated through the promotion of repair gene transcription, resulting in improved cardiac repair and function post-MI. Notably, lactylation at the H3K18 site plays a central role in this physiological process [20]. Furthermore, lactylated histone H3K18 shows promise as a potential biomarker for assessing the severity and predicting infectious shock, while also regulating the anti-inflammatory function of macrophages [37]. Studies on cerebral IRI have revealed that lactate dehydrogenase A mediates histone lactylation induced by cerebral IRI by targeting HMGB1, thereby triggering pyroptosis [38]. These findings provide new insights into the complex role of lactylation in various physiological and pathological processes associated with cerebral IRI.
Systematic analysis of current evidence has revealed several critical aspects regarding histone lactylation modification. While extensively studied modification sites such as H3K18 have been well-characterized across various pathological conditions [20, 37, 65], investigation of other histone sites remains insufficient. Although this site-specific investigative approach has yielded valuable insights, it potentially limits comprehensive understanding of the global impact of histone lactylation modifications. The temporal dynamics of histone lactylation modifications warrant particular attention, as exemplified in myocardial infarction, where H3K18 lactylation demonstrates distinct patterns during various phases of cardiac repair [20]. However, comparable temporal analyses in other disease contexts remain limited, particularly in IRI, where intervention timing is crucial for therapeutic efficacy. Moreover, current research faces several methodological challenges: 1. Excessive reliance on correlative analyses between specific histone site lactylation levels and disease outcomes, with insufficient investigation of causal relationships; 2. Predominant focus on endpoint measurements rather than temporal trajectory analyses; 3. Limited understanding of tissue-specific variations in histone lactylation patterns, suggesting complex regulatory mechanisms requiring further investigation. However, given the fundamental role of energy metabolism in biological processes and current research evidence, investigations focusing on lactate and histone lactylation modifications represent promising research directions for developing therapeutic strategies against metabolic diseases, particularly malignancies.
Nonhistone lactylation
Owing to technological advancements, notably the advent of liquid chromatography-mass spectrometry, the research focus has expanded from histone lactylation to encompass nonhistone lactylation [55, 56]. A 2020 study revealed that proteomic analysis identified 273 lactylation sites across 166 proteins within Botrytis cinerea, a harmful necrotrophic fungal pathogen. Intriguingly, nearly two-thirds of these lactylated proteins were observed to be cytoplasmic [69]. Sung et al.’s investigation into liver Kupffer cells disclosed 298 lactylation sites in 181 proteins, predominantly localized in the nucleus (60%), cytoplasm (28%), mitochondria (4%), cell projections (3%), and chromosomes (3%) [61]. These findings suggest that, beyond histone lactylation, a vast array of nonhistone proteins are capable of undergoing lactylation. Thus, advancing research is expected to progressively uncover the complex relationship between nonhistone lactylation and the development and progression of diseases.
The current research on lactylation in nonhistone proteins predominantly focuses on its implications in tumors, liver and kidney injuries, as well as heart failure [21, 24, 62, 70,71,72]. Similar to histone lactylation, nonhistone lactylation’s correlation with various cancer-related diseases has been established, encompassing colon cancer, hepatocellular carcinoma, lung cancer, gastric cancer, prostate cancer, colorectal cancer, and glioblastoma [21, 55, 70, 71, 73,74,75]. Crucial in tumor immune escape, tumor-infiltrating myeloid cells have been shown to be significantly affected by lactylation. Recent studies have identified that lactylation at K281 and K345 sites of methyltransferase-like 3(METTL3) can effectively modulate the epigenetic modification of its associated RNA N6-methyladenosine, thereby inducing immunosuppression in these cells [55]. Additionally, the liver, as the primary metabolic organ for lactate, has been the subject of numerous studies highlighting lactate accumulation-mediated lactylation in liver tumors [21, 76,77,78]. For instance, lactylation of adenylate kinase 2 at the K28 site has been found to inhibit its function, thereby facilitating proliferation and metastasis in hepatitis B virus-related hepatocellular carcinoma cells [21].
Notably, recent research has identified novel nonhistone lactate transferases, expanding our understanding of lactylation mechanisms. Mitochondrial alanyl-tRNA synthetase 1 (AARS1) demonstrates a strong affinity for lactate and catalyzes the formation of lactate-AMP, subsequently transferring lactate to lysine receptor residues. This process induces lactylation of P53 and promotes tumor development by regulating mechanisms such as the Hippo pathway [79, 80]. Similarly, AARS2 has been found to regulate cell metabolism through lactylation, specifically by limiting the influx of acetyl-CoA in pyruvate and fatty acid oxidation processes, ultimately inhibiting oxidative phosphorylation [81]. These findings greatly enrich our understanding of the research field of nonhistone “writers.”
Additionally, a significant link has been established between nonhistone lactylation and various non-neoplastic diseases. Pyruvate kinase M2, a critical regulator of metabolic adaptations in pro-inflammatory macrophages, has been a key focus in this area. Research by Wang et al. revealed that increased lactylation levels of pyruvate kinase M2 at the K62 site due to lactate exposure inhibits its transition from tetramer to dimer. This inhibition enhances its pyruvate kinase activity and decreases its nuclear distribution, consequently facilitating the transition of pro-inflammatory macrophages to a reparative phenotype [56].
It is worth noting that there is a significant association between nonhistone lactylation and IRI. Research has revealed that endothelial-mesenchymal transition plays a central role in the pathological process of cardiac fibrosis. Specifically, lactate can accelerate the endothelial-mesenchymal transition process after myocardial infarction, thereby exacerbating cardiac fibrosis and worsening cardiac function, with Snail1 lactylation playing a key role in this complex mechanism [82]. Additionally, in the field of heart failure research, lactylation of α-myosin heavy chain has been observed to exhibit dynamic regulation characteristics during the progression of heart failure. This modification is not only an important determinant of overall cardiac structure and function but also provides us with a new approach to alleviate heart failure symptoms - by enhancing the lactylation level at the K1897 site of α-myosin heavy chain [62]. Similarly, in the context of liver IRI, nonhistone lactylation also exhibits unique biological effects. Studies have shown that heat shock protein A12A (HSPA12A) in hepatocytes can effectively alleviate liver IRI pathological damage by inhibiting glycolysis-mediated HMGB1 lactylation and hepatocyte secretory activity, thereby weakening the chemotaxis and activation ability of macrophages. This finding suggests that targeted therapy for hepatocyte HSPA12A may show potential in future clinical treatment of liver IRI [83].
Overall, accumulated evidence has elucidated multiple mechanisms underlying nonhistone lactylation regulation. First, metabolic regulation occurs through direct modification of metabolic enzymes affecting their activity and stability, as demonstrated in the glycolysis pathway regulation [56]. Second, transcriptional regulation involves lactylation modification of transcription factors that modulate gene expression programs, particularly evident in inflammatory responses [28]. Third, structural regulation encompasses modifications of structural proteins that influence cellular architecture and function, as exemplified by cardiac myosin modifications [62]. In the context of IRI, several distinctive patterns have emerged: cell-type specificity, where different cell populations exhibit distinct lactylation responses, notably demonstrated in hepatocyte HSPA12A-mediated regulation of HMGB1 lactylation [83]; temporal dynamics, where lactylation modification patterns demonstrate dynamic alterations during various stages of injury and repair [82]; and therapeutic implications, where targeting lactylation modifications shows therapeutic potential [62, 83], although clinical translation remains challenging.
Furthermore, current research faces several significant limitations in the field of protein lactylation. A primary technical constraint involves the detection and quantification of in vivo lactylation modifications. Sun et al. noted that current mass spectrometry-based analytical methods can identify only a subset of lactylation sites, potentially overlooking low-abundance modifications [61]. Additionally, understanding of tissue-specific regulatory patterns remains insufficient. Although lactylation sites have been identified across various tissues, comprehension of tissue-specific regulatory mechanisms remains limited. For instance, Hong et al.’s comprehensive analysis of hepatocellular carcinoma revealed unique lactylation patterns, though the underlying regulatory mechanisms remain incompletely understood [77]. Similarly, Sun et al.’s investigations of Kupffer cells demonstrated distinct cellular lactylation profiles [61], revealing notable discrepancies with hepatocellular findings [77], thus necessitating further investigation. Furthermore, functional impacts of lactylation modifications demonstrate significant context dependency under different pathological conditions. Yang et al.’s research demonstrated that lactylation promotes metabolic adaptation in hepatocellular carcinoma [21], while Xiong et al.’s study revealed lactylation’s involvement in immunosuppression mediated by tumor-infiltrating myeloid cells [55]. These apparently contradictory findings underscore the necessity for careful evaluation of context-specific functions when developing therapeutic strategies. Moreover, while some studies indicate beneficial effects of lactylation under certain conditions [20], others suggest potential adverse effects [28], emphasizing the importance of comprehensive investigation to understand these contrasting phenomena.
In the context of IRI, diminished tissue perfusion during the ischemic phase leads to enhanced anaerobic glycolysis and subsequent lactate accumulation. Such metabolic shifts prompt the lactylation of both histone and nonhistone proteins, impacting the onset and progression of IRI. Future research endeavors will aim to further clarify the interplay between lactylation and IRI, building upon the existing body of evidence. Next, we will present a comprehensive analysis of the lactylation sites identified to be closely associated with IRI (Fig. 5, Table 2). This exploration will delve into the specific locations and their relevance to the pathophysiology of IRI, highlighting the critical role these sites play in the progression and manifestation of the injury.
Lactylation in both histone and nonhistone proteins is a critical factor in several diseases linked to ischemia-reperfusion injury. This process significantly impacts organs and tissues such as the heart, liver, kidney, brain, and others, underscoring its importance in the pathophysiology of these conditions. Created with Figdraw.
MI
Annually, acute MI affects approximately 7 million individuals globally. Despite considerable advancements in therapeutic and diagnostic techniques, MI continues to be a predominant cause of mortality worldwide [84]. MI, commonly known as a heart attack, occurs due to plaque accumulation in the coronary arteries, leading to diminished blood flow and oxygen supply to the heart [85]. Research has elucidated that the pathogenesis and progression of MI are governed by a complex array of mechanisms. These include the generation of reactive oxygen species and pro-inflammatory mediators, activation of immune responses, stimulation of both angiogenesis and lymphangiogenesis, alterations in myocardial electrophysiology, and induction of cellular apoptosis [84].
Recent advancements in medical research suggest a significant link between epigenetic regulation and the pathogenesis of myocardial IRI, positing epigenetics as a potential novel therapeutic target for mitigating or preventing IRI [86]. Epigenetic modifications of histones involve a spectrum of processes such as methylation, acetylation, phosphorylation, ubiquitylation, sumoylation, among others [87]. Current investigations into myocardial IRI predominantly explore the dynamic and reversible nature of methylation and acetylation alterations [86]. For instance, studies suggest that the cardioprotective effect of H3K9 methylation may stem from its role in reducing inflammation by inhibiting the MAPK/NF-κB signaling pathway [86]. Furthermore, the inflammatory response following AMI is crucial in determining the extent of MI. Persistent pro-inflammatory reactions can lead to adverse post-MI left ventricular remodeling, highlighting inflammation as a vital therapeutic target to enhance outcomes post-AMI [88]. Interestingly, postconditioning with lactate-enriched blood has shown good protective effects on ST-segment elevation MI [89]. These findings emphasize the important role of histone epigenetic modifications and lactate-mediated inflammatory regulation in the pathogenesis and treatment of MI.
In 2022, Wang et al. elucidated that histone lactylation orchestrates the anti-inflammatory and pro-angiogenic dual functions of monocyte-macrophages. This process aids in reparative gene transcription, fostering a conducive environment for cardiac repair and enhancing cardiac function post-MI [20]. A subsequent study revealed a novel role of lactate in augmenting cardiac fibrosis and exacerbating cardiac dysfunction. This effect is mediated through the promotion of endothelial-to-mesenchymal transition, a key player in cardiac fibrosis, following MI [82]. Moreover, a clear association between lactylation modification and both heart failure and atherosclerosis has been established. The interaction between α-myosin heavy chain and Titin is essential for maintaining cardiac structure and contraction. The dynamic lactylation of α-myosin heavy chain is a critical determinant of cardiac integrity and function. A reduction in intracellular lactate levels, primarily due to excessive efflux and consumption by cardiomyocytes, contributes significantly to diminished lactylation at the K1897 site of α-myosin heavy chain during myocardial injury [62].
Furthermore, recent studies have elucidated significant effects of exercise on improving cardiac function and promoting metabolism. A key mechanism involves exercise training inhibiting YTHDF2 lactylation, thereby effectively preventing acute injury and pathological remodeling in myocardial IRI [90]. Additionally, HSPA12A has been shown to play a crucial role in myocardial protection by maintaining aerobic glycolysis homeostasis in cardiomyocytes and regulating histone 3 lactylation, thus alleviating myocardial IRI [91]. These findings underscore the complex and critical role of lactylation in myocardial IRI, acting as a double-edged sword with drastically different effects under various signaling pathways or intervention conditions. Further in-depth research is necessary to elucidate the specific mechanisms of lactylation in myocardial IRI.
Cardiomyocytes are remarkably sensitive to changes in energy metabolism, and epigenetic modifications play an instrumental role in the initiation and progression of MI. With ongoing research, it is anticipated that more lactylation sites, whether on histone or nonhistone proteins, will be identified in the context of MI.
Liver IRI
IRI significantly contributes to liver damage during surgical procedures, such as hepatic resection and liver transplantation. It is a primary factor in graft dysfunction and post-transplantation liver failure [4]. Liver IRI involves a complex interplay of cellular and molecular mechanisms, leading to an exacerbated inflammatory response [92]. Liver-resident macrophages and recruited cells produce pro-inflammatory signals, including reactive oxygen species, cytokines, chemokines, proteases, and lipid mediators. These signals trigger the inflammasome, intensifying the inflammatory response and accelerating hepatocyte necrosis and apoptosis [4, 92, 93].
PTMs profoundly affect almost all proteins, playing a vital role in preserving the functionality and diversity of the proteome. The role of PTMs in the onset and progression of liver IRI has been substantiated by numerous studies, with ubiquitination standing out as a key area of research [94, 95]. For example, the NF-κB-mediated inflammatory response is crucial in liver IRI, and the autopolyubiquitination of K63-linked tumor necrosis factor receptor-associated factor 6 is essential for NF-κB activation. OTU domain protein 4, acting as a deubiquitinating enzyme, interacts with tumor necrosis factor receptor-associated factor 6 to reduce its K63 autopolyubiquitination, thereby mitigating liver ischemia-reperfusion injury [94].
In addition to ubiquitination, histone-mediated PTMs are also implicated in liver IRI. Notably, the SIRT1 can attenuate liver IRI in mice. This mitigation is achieved by inhibiting the XBP1/NLRP3 inflammatory pathway through miR-182 activation, while SIRT6 offers protection against liver IRI by repressing apoptosis and autophagy-related cell death [96, 97]. Liver IRI is characterized by two interlinked phases: ischemia-induced hepatocyte injury, leading to the release of damage-associated molecular patterns, and subsequent recruitment of immune cells, initiating an inflammatory cascade that exacerbates hepatocyte damage. HMGB1 is a key damage-associated molecular patterns in this process. Research has shown that HSPA12A, an unconventional member of the HSP70 family, inhibits HMGB1 lactylation and secretion from hepatocytes, mediated by glycolysis. This inhibition curtails macrophage chemotaxis and inflammatory activation, ultimately offering protection against liver IRI [83]. Lactylation significantly increases HMGB1 release from hepatocytes, a key component of damage-associated molecular patterns, actively mediating subsequent liver IRI. Moreover, HMGB1 serves as an indispensable intermediate bridge in HSPA12A’s protection against liver IRI.
These observations strongly suggest that PTMs of both histones and non-histones may play pivotal roles in liver IRI pathogenesis. While research on the connection between lactylation and liver IRI is still in its infancy, the liver’s unique position as the core organ of lactate metabolism suggests that future advancements will likely reveal more lactate-mediated lactylation sites closely related to liver IRI.
Septic shock
The study of septic shock remains a critical area of interest, owing to its propensity for inducing severe complications and mortality. Frequently, the host’s pro-inflammatory response, aimed at eliminating the invading pathogens, may inadvertently inflict collateral tissue damage. This is especially evident during episodes of severe sepsis. In contrast, the anti-inflammatory response is crucial in mitigating both localized and systemic tissue injury. Nevertheless, its overactivation or inappropriate engagement can increase the susceptibility to secondary infections. Furthermore, factors such as innate immune responses, coagulopathy, the modulation of the anti-inflammatory/pro-inflammatory equilibrium, and immunosuppression, collectively play a role in the onset and progression of septic shock, potentially leading to organ dysfunction [98].
The pathogenesis of septic shock is complex and multi-dimensional. Studies have shown that PTMs such as ubiquitination and acetylation are implicated in the initiation and progression of this condition [99, 100]. For instance, a key element in the innate immune response is the inflammation mediated by Toll-like receptors. The E3 ligase RNF99 plays a critical role by interacting with and targeting TAK1-binding protein 2 for degradation. TAK1-binding protein 2 is a regulatory protein of the kinase TAK1, and its degradation occurs via the K48-linked ubiquitin-proteasomal pathway at lysine 611. This interaction is instrumental in modulating the Toll-like receptors-mediated inflammatory response [99]. Additionally, circulating histones such as H2B, H3, and citrullinated H3 have been identified as valuable biomarkers for the early diagnosis of sepsis and the assessment of its severity [101, 102].
Septic shock, a systemic disease, can profoundly affect multiple organ systems. Recent studies have highlighted the role of SIRT3 downregulation in mediating the hyperacetylation and subsequent inactivation of the pyruvate dehydrogenase E1 component subunit alpha. This alteration leads to excessive lactate production in renal tubular epithelial cells, contributing to the pathogenesis of septic shock. The resultant increase in lactate levels can induce lactylation of the mitochondrial fission 1 protein, exacerbating sepsis-induced acute kidney injury. Conversely, reducing lactate levels and mitochondrial fission 1 protein lactylation may attenuate this injury [72]. Further research has identified lactylation as a novel PTM in peripheral blood monocytes, observed in both healthy individuals and critically ill patients. Notably, patients with septic shock exhibit a significantly higher relative density of this modification. Lactylation at H3K18 is believed to regulate the expression of inflammatory cytokines and promote the overexpression of arginase-1, thereby enhancing the anti-inflammatory response of macrophages during sepsis [37].
These findings underscore the critical role of histones and their PTMs in both the detection and progression of septic shock. This is further supported by the strong correlation between lactate levels, including their temporal changes, and the morbidity and mortality associated with sepsis, as well as tissue hypoxia and hypoperfusion [103]. These research results indicate that lactylation has potential as a treatment target for sepsis-induced injury. Given that sepsis is a systemic injury disease, the high expression of certain lactylation sites may serve as diagnostic and predictive indicators, expanding the application scope of lactylation in sepsis research. Consequently, future research endeavors in septic shock may benefit from a focus on lactate and its associated lactylation processes.
Cerebral IRI
Stroke, ranking as the second leading cause of death worldwide, results in a spectrum of severe cerebral disorders, leading to mortality and varying degrees of impairment [104, 105]. Reperfusion of ischemic brain tissue exacerbates oxidative stress and triggers an increased release of pro-inflammatory cytokines. This initiates a series of pathological cascades, culminating in apoptosis, blood-brain barrier disruption, cerebral edema, and hemorrhagic transformation [105]. The progression of cerebral IRI is multifactorial, involving activation of microglia and astrocytes, immune cell infiltration, platelet activation, and various cell death pathways such as apoptosis, necrosis, and ferroptosis. Among these, the inflammatory cascade is particularly notable as a central mechanism exacerbating cerebral IRI [105, 106].
Studies have shown that following focal and global cerebral ischemia, there is an elevation in ubiquitin-protein conjugates within the brain. Post-ischemic ubiquitination is rapidly induced upon reperfusion and is transiently observed in potentially viable neurons within the peri-infarct area [107]. Recent advances in molecular biology reveal that gene expression can be regulated by PTMs without altering the genetic code’s chemical structure. In this context, HDACs play a critical role. These enzymes repress gene expression by removing acetyl groups from lysine residues on histones, leading to chromatin compaction. The pharmacological inhibition of HDACs has been explored for activating protective genes, and their role in the mechanism of cerebral IRI has been studied [108]. Moreover, preclinical evidence suggests that HDAC inhibitors could be a promising and cost-effective therapeutic strategy for enhancing neurological and cardiac outcomes post-IRI. This therapeutic potential is primarily achieved by promoting angiogenesis, neurogenesis, and stem cell migration, which collectively contribute to a marked reduction in infarct volume and enhanced functional recovery in experimental models of cerebral ischemia [109, 110].
A recent proteomic study revealed that in a rat model of acute ischemic stroke, lactylation of cortical proteins was modified at 1003 sites across 469 proteins [111]. Additionally, research indicated that treating N2a cells with oxygen-glucose deprivation/reoxygenation upregulated HMGB1 and lactate expression and increased histone lactylation levels. Lactate dehydrogenase A modulates pyroptosis induced by histone lactylation in cerebral ischemia through HMGB1. Inhibiting lactate dehydrogenase A expression has been shown to reverse this pyroptosis [38]. Interestingly, lactylation’s role extends beyond cerebral ischemic conditions. It also appears to be associated with cerebral hemorrhagic diseases. Intracerebral hemorrhage, which results from increased vascular fragility and rupture in the non-traumatic brain parenchyma, has been strongly linked to ferroptosis. A study demonstrated that lactylation of METTL3 increased in heme-treated PC12 cells. This enhancement led to greater protein stability and elevated METTL3 expression, which in turn upregulated the m6A level and mRNA expression of the transferrin receptor, further inducing ferroptosis in the PC12 cells [112].
Recent findings reveal that various molecules and pathways, including nuclear receptor coactivator 4, astrocyte-derived lactate, low-density lipoprotein receptor-related protein 1, and cGAS signaling, can influence cerebral IRI progression through unique lactylation mechanisms or by directly regulating overall lactylation levels [113,114,115,116]. Notably, lactylation also acts as a key mediator for some drugs in cerebral IRI protection, such as Buyang Huanwu Decoction, which effectively inhibits cerebral IRI deterioration by suppressing extensive lysine lactylation and specific H3K18 lactylation [117].
These findings highlight lactylation’s crucial role in cerebral IRI, demonstrating distinct bipolar characteristics with potential benefits and non-negligible drawbacks. Furthermore, lactylation shows promise as a key intermediate mediator for certain drugs to achieve cerebral IRI protection. Given that glucose metabolism is the brain’s primary energy source, an adequate blood supply is essential for sustaining brain function. During cerebral ischemia, the brain predominantly relies on anaerobic glycolysis for energy, resulting in the accumulation of lactate, which mediates an increase in lactylation. Notably, there is evident reciprocal regulation between lactylation and inflammation. This suggests that targeting lactylation could provide promising intervention strategies for cerebral IRI.
Conclusions and perspectives
Adequate blood supply is essential for sustaining normal bodily functions, and IRI has been shown to play a pivotal role in the onset and progression of various diseases. These include myocardial infarction, hepatic IRI, septic shock, and cerebral IRI, as evidenced in studies [37, 83, 86, 118]. During tissue ischemia, cellular energy metabolism undergoes a shift, with glycolysis becoming the dominant source of energy. This metabolic shift can lead to increased lactate production and accumulation, consequently resulting in increased lactylation.
Lactylation, a PTM first identified by Professor Zhao Yingming’s research team in 2019, has emerged as a key focus in PTM research [16]. Lactylation predominantly involves modifications on lysine residues of histone or nonhistone proteins, triggered by lactate accumulation. This modification has been linked to the development and progression of various diseases, such as melanoma, myocardial infarction, hepatocellular carcinoma, Alzheimer’s disease, and nonalcoholic fatty liver disease [18,19,20,21,22,23,24]. Additionally, research has revealed a complex interplay between lactylation and inflammation [25,26,27,28]. These regulatory mechanisms extend beyond energy metabolism to encompass broader biological significance. Recent investigations have demonstrated that during IRI, lactate accumulation and subsequent lactylation effects function as critical molecular bridges between metabolic stress and cellular responses [37, 83, 86, 118]. The interaction between lactylation modifications and inflammatory regulatory mechanisms exhibits complex bidirectional characteristics. Specifically, lactylation directly modulates the activity of key inflammatory mediators, including NF-κB and STAT proteins, enabling precise regulation of inflammatory responses [55, 67]. Additionally, lactylation-mediated chromatin modifications establish specific epigenetic environments that modulate inflammatory gene expression in a context-dependent manner [16, 67, 68]. Furthermore, lactylation of metabolic enzymes, particularly PKM2 and IDH3G, significantly influences cellular energy metabolism, thereby affecting immune cell function and inflammatory responses [56, 119].
The Warburg effect, pivotal in advancing our understanding of glycolysis, has clarified that lactate production can occur under both aerobic and anaerobic conditions. This phenomenon underscores the need for further investigation into the regulatory roles of enzymes, such as HDACs, in histone PTMs. A critical aspect of this research focuses on the balance between histone acetylation and lactylation. Studies have shown that histone lactylation and acetylation can compete for the epigenetic modification of lysine residues, thereby influencing the levels of lactate and acetyl-CoA. Further research is essential to determine whether pyruvate, at the glycolytic pathway’s terminus, preferentially contributes to lactate or acetyl-CoA synthesis, impacting subsequent cellular developmental processes [60]. Moreover, lactylation and acetylation often share writer and eraser mechanisms, playing antagonistic roles in various diseases. Studies have shown that under lactate-deficient conditions, H3K18 lactylation is significantly reduced, correlating with changes in gene expression patterns, while H3K27 acetylation shows minimal correlation. These findings suggest that lactate may be more inclined to regulate early embryonic development by affecting H3K18 lactylation rather than H3K27 acetylation [120].
Moreover, in the context of IRI, substantial evidence indicates significant crosstalk between lactylation and other PTMs. During sepsis, macrophages utilize monocarboxylate transporters for extracellular lactate uptake, promoting HMGB1 lactylation through p300/CBP-dependent mechanisms. Concurrent with this process, lactate induces HMGB1 acetylation through dual pathways: Hippo/YAP-mediated SIRT1 and β-arrestin2 inhibition, and G-protein-coupled receptor 81 (GPR81)-mediated recruitment of p300/CBP acetyltransferase. The resultant lactylated/acetylated HMGB1, released via macrophage-derived exosomes, subsequently enhances endothelial cell permeability. Notably, in vivo reduction of lactate production or inhibition of GPR81-mediated signaling pathways significantly decreases circulating exosomal HMGB1 levels, thereby improving survival outcomes in polymicrobial sepsis [28]. These observations demonstrate that individual proteins can undergo simultaneous lactylation and acetylation modifications, potentially generating synergistic effects. Although comprehensive understanding of the competitive mechanisms between these PTMs requires further investigation, current evidence strongly suggests intimate regulatory connections between lactylation and other PTMs, particularly acetylation. Future research is expected to reveal increasingly complex and diverse interactions between lactylation and various PTMs networks. These findings emphasize the close connection between lactylation and other PTMs, particularly acetylation. As research deepens, we have reason to believe that more evidence will reveal the complex and diverse interactions between lactylation and other PTMs.
Notably, the lactylation of the nonhistone protein YY1 in microglia has been identified as a key factor in retinal neovascularization, primarily through upregulating FGF2 expression [121]. Concurrently, studies have shown that evodiamine effectively inhibits lactate-induced angiogenesis in prostate cancer cells. This inhibition occurs by modulating histone lactylation and HIF1A expression, which consequently enhances Sema3A transcription and suppresses PD-L1 expression [122]. These results clearly reveal that lactylation also has significant regulatory effects in angiogenesis, expanding its application prospects in IRI to early angiogenesis and blood supply restoration. These discoveries enhance our comprehensive understanding of the interaction mechanisms between lactylation and IRI.
It is worth noting that the interactions between different lactylation sites in histones are equally thought-provoking. To date, numerous lactylation sites have been identified on histones, covering almost all five histone subtypes, with H3K18 being particularly well-studied. In various diseases, multiple sites are modified simultaneously [123]. Recent studies suggest synergistic effects of H3K18 and H3K9 lactylation in regulating CD8 T-cell effector function [124], and H3K14 and H3K9 lactylation in promoting calcification in calcific aortic valve disease [125]. Therefore, it is particularly important to deeply explore the differences and connections between different modification sites on histones. Furthermore, lactylation also regulates various enzyme activities or acts as downstream pathways of enzymes, including aldehyde dehydrogenase 1A3, lactate dehydrogenase A and B, and HDAC2 [126,127,128,129]. These pathways further expand the modes and ranges of lactylation’s effects in different diseases, emphasizing the important role of lactylation in regulating disease progression. However, current investigations of histone lactylation modifications have predominantly focused on specific modification sites, particularly H3K18 [20, 37, 65], while studies examining modifications at other histone sites remain limited. Although this site-specific investigative approach has provided valuable mechanistic insights, it potentially constrains comprehensive understanding of the global effects of histone lactylation modifications. Moreover, the temporal dynamics of histone lactylation modifications necessitate more extensive investigation to fully elucidate their biological significance.
Overall, in the context of IRI, both anaerobic glycolysis during ischemia and the exacerbation of cellular functional damage during reperfusion are instrumental in lactate production and accumulation. This lactate subsequently induces histone or nonhistone lactylation, playing a pivotal role in the progression of IRI. Despite these insights, several aspects of lactylation remain elusive. These include the complex equilibrium of various PTMs on histone lysine residues, the potential interplay between histone and nonhistone lactylation and their respective impacts on cellular function, and their distinct roles in the pathophysiology of IRI. Notably, recent studies have unveiled significant interactions between histone and nonhistone lactylation modifications. These distinct PTMs share several crucial regulatory enzymes, including P300 as a writer enzyme [16, 18, 121] and HDACs/SIRTs functioning as erasers [18, 59, 78]. The recent characterization of novel acetyltransferases AARS1 and AARS2 provides additional evidence supporting coordinated regulatory mechanisms between these PTMs [79,80,81]. However, the precise molecular interactions between these modification types and their detailed coordination mechanisms in cellular responses remain incompletely understood, particularly within the context of IRI. Enhanced understanding of these intricate interactions may reveal novel therapeutic strategies targeting lactylation modifications. Further investigation of the molecular mechanisms governing lactylation writers and erasers is essential for developing a comprehensive understanding of this PTM system and elucidating complex interactions among various PTMs. Notably, the regulatory network between lactylation and acetylation modifications likely extends beyond currently identified factors such as HDACs, histone acetyltransferase p300, and AARS1/2, suggesting the potential existence of additional regulatory components awaiting discovery.
Moreover, an in-depth exploration of inter-organ communication in response to IRI represents a highly promising research direction. Of particular interest is the rapid interaction between intestinal and cerebral IRI mediated by neural circuits. Notably, cerebral IRI has been shown to rapidly induce secondary intestinal IRI, a finding that has opened up novel avenues for investigation in this field [130]. Advances in technological fields, particularly in proteomics, are expected to facilitate the increased identification of lactylation sites, especially nonhistone ones. This progress will likely open new avenues for research and provide innovative strategies for intervention. Such developments are anticipated to significantly enhance our understanding of disease pathogenesis and inform the development of therapeutic approaches for related conditions.
References
Lier H, Bernhard M, Hossfeld B. Hypovolämisch-hämorrhagischer Schock [Hypovolemic and hemorrhagic shock]. Anaesthesist. 2018;67:225–44.
Sunder-Plassmann P. Durchblutungsschäden und ihre Behandlung [Reperfusion injury and its treatment]. Dermatol Wochenschr.1952;125:224–34.
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46:1650–67.
Jiménez-Castro MB, Cornide-Petronio ME, Gracia-Sancho J, Peralta C. Inflammasome-mediated inflammation in liver ischemia-reperfusion injury. Cells. 2019;8:1131.
Li Z, Ludwig N, Thomas K, Mersmann S, Lehmann M, Vestweber D, et al. The pathogenesis of ischemia-reperfusion induced acute kidney injury depends on renal neutrophil recruitment whereas sepsis-induced AKI does not. Front Immunol. 2022;13:843782.
Tang Q, Dong C, Sun Q. Immune response associated with ischemia and reperfusion injury during organ transplantation. Inflamm Res. 2022;71:1463–76.
Pretzsch E, Nieß H, Khaled NB, Bösch F, Guba M, Werner J, et al. Molecular mechanisms of ischaemia-reperfusion injury and regeneration in the liver-shock and surgery-associated changes. Int J Mol Sci. 2022;23:12942.
Song R, Dasgupta C, Mulder C, Zhang L. MicroRNA-210 controls mitochondrial metabolism and protects heart function in myocardial infarction. Circulation. 2022;145:1140–53.
Mao Z, Tian L, Liu J, Wu Q, Wang N, Wang G, et al. Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomedicine. 2022;101:154111.
Przykaza Ł. Understanding the connection between common stroke comorbidities, their associated inflammation, and the course of the cerebral ischemia/reperfusion cascade. Front Immunol. 2021;12:782569.
Ye J, Hu X, Wang Z, Li R, Gan L, Zhang M, et al. The role of mtDAMPs in the trauma-induced systemic inflammatory response syndrome. Front Immunol. 2023;14:1164187.
Liao S, Luo J, Kadier T, Ding K, Chen R, Meng Q. Mitochondrial DNA release contributes to intestinal ischemia/reperfusion injury. Front Pharmacol. 2022;13:854994.
Peoc’h K, Nuzzo A, Guedj K, Paugam C, Corcos O. Diagnosis biomarkers in acute intestinal ischemic injury: so close, yet so far. Clin Chem Lab Med. 2018;56:373–85.
Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118:691–728.
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7:305.
Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–80.
Narita T, Weinert BT, Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol. 2019;20:156–74.
Wang JH, Mao L, Wang J, Zhang X, Wu M, Wen Q, et al. Beyond metabolic waste: lysine lactylation and its potential roles in cancer progression and cell fate determination. Cell Oncol. 2023;46:465–80.
Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, et al. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22:85.
Wang N, Wang W, Wang X, Mang G, Chen J, Yan X, et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ Res. 2022;131:893–908.
Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S, et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. 2023;5:61–79.
Lv X, Lv Y, Dai X. Lactate, histone lactylation and cancer hallmarks. Expert Rev Mol Med. 2023;25:e7.
Pan RY, He L, Zhang J, Liu X, Liao Y, Gao J, et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022;34:634–648.e6.
Gao R, Li Y, Xu Z, Zhang F, Xu J, Hu Y, et al. Mitochondrial pyruvate carrier 1 regulates fatty acid synthase lactylation and mediates treatment of nonalcoholic fatty liver disease. Hepatology. 2023;78:1800–15.
Cheng X, Wang K, Zhao Y, Wang K. Research progress on post-translational modification of proteins and cardiovascular diseases. Cell Death Discov. 2023;9:275.
Xie Y, Hu H, Liu M, Zhou T, Cheng X, Huang W, et al. The role and mechanism of histone lactylation in health and diseases. Front Genet. 2022;23:13.
Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci USA. 2020;117:30628–38.
Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022;29:133–46.
Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–51.
Aimo A, Borrelli C, Giannoni A, Pastormerlo LE, Barison A, Mirizzi G, et al. Cardioprotection by remote ischemic conditioning: mechanisms and clinical evidences. World J Cardiol. 2015;7:621–32.
Arnold JR, P Vanezis A, Rodrigo GC, Lai FY, Kanagala P, Nazir S, et al. Effects of late, repetitive remote ischaemic conditioning on myocardial strain in patients with acute myocardial infarction. Basic Res Cardiol. 2022;117:23.
Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714–22.
Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17:773–89.
Chen CL, Zhang L, Jin Z, Kasumov T, Chen YR. Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2022;322:C12–C23.
Morciano G, Bonora M, Campo G, Aquila G, Rizzo P, Giorgi C, et al. Mechanistic role of mPTP in ischemia-reperfusion injury. Adv Exp Med Biol. 2017;982:169–89.
Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599:1745–57.
Chu X, Di C, Chang P, Li L, Feng Z, Xiao S, et al. Lactylated histone H3K18 as a potential biomarker for the diagnosis and predicting the severity of septic shock. Front Immunol. 2022;12:786666.
Yao X, Li C. Lactate dehydrogenase A mediated histone lactylation induced the pyroptosis through targeting HMGB1. Metab Brain Dis. 2023;38:1543–53.
Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64.
Singh M, Afonso J, Sharma D, Gupta R, Kumar V, Rani R, et al. Targeting monocarboxylate transporters (MCTs) in cancer: how close are we to the clinics? Semin Cancer Biol. 2023;90:1–14.
Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35:101454.
Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM, et al. A lactate-induced response to hypoxia. Cell. 2015;161:595–609.
Zhang W, Wang G, Xu ZG, Tu H, Hu F, Dai J, et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell. 2019;178:176–89.e15.
Liu W, Wang Y, Bozi LHM, Fischer PD, Jedrychowski MP, Xiao H, et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature. 2023;616:790–7.
Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, et al. lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015;13:e1002202.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
Manosalva C, Quiroga J, Hidalgo AI, Alarcón P, Anseoleaga N, Hidalgo MA, et al. Role of lactate in inflammatory processes: friend or foe. Front Immunol. 2022;12:808799.
Luo Y, Li L, Chen X, Gou H, Yan K, Xu Y. Effects of lactate in immunosuppression and inflammation: progress and prospects. Int Rev Immunol. 2022;41:19–29.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Nistri S, Sassoli C, Bani D. Notch signaling in ischemic damage and fibrosis: evidence and clues from the heart. Front Pharmacol. 2017;8:187.
Bonaventura A, Montecucco F, Dallegri F. Cellular recruitment in myocardial ischaemia/reperfusion injury. Eur J Clin Invest. 2016;46:590–601.
Koyama T. Postconditioning with lactate-enriched blood for reducing lethal reperfusion injury in humans. J Cardiovasc Transl Res. 2023;16:793–802.
Yang J, Luo L, Zhao C, Li X, Wang Z, Zeng Z, et al. A positive feedback loop between inactive VHL-triggered histone lactylation and PDGFRβ signaling drives clear cell renal cell carcinoma progression. Int J Biol Sci. 2022;18:3470–83.
Pan L, Feng F, Wu J, Fan S, Han J, Wang S, et al. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270.
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82:1660–77.e10.
Wang J, Yang P, Yu T, Gao M, Liu D, Zhang J, et al. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int J Biol Sci. 2022;18:6210–25.
Zhang B, Li F, Shi Y, Ji C, Kong Q, Sun K, et al. Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury. CNS Neurosci Ther. 2024;30:e70028.
Huang J, Wang X, Li N, Fan W, Li X, Zhou Q, et al. YY1 lactylation aggravates autoimmune uveitis by enhancing microglial functions via inflammatory genes. Adv Sci. 2024;11:e2308031.
Moreno-Yruela C, Zhang D, Wei W, Bæk M, Liu W, Gao J, et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci Adv. 2022;8:eabi6696.
Dai X, Lv X, Thompson EW, Ostrikov KK. Histone lactylation: epigenetic mark of glycolytic switch. Trends Genet. 2022;38:124–7.
Sung E, Sim H, Cho YC, Lee W, Bae JS, Tan M. Global profiling of lysine acetylation and lactylation in Kupffer cells. J Proteome Res. 2023;22:3683–91.
Zhang N, Zhang Y, Xu J, Wang P, Wu B, Lu S, et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023;33:679–98.
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang Y, et al. Overview of histone modification. Adv Exp Med Biol. 2021;1283:1–16.
Xin Q, Wang H, Li Q, Liu S, Qu K, Liu C, et al. Lactylation: a passing fad or the future of posttranslational modification. Inflammation. 2022;45:1419–29.
Li X, Yang N, Wu Y, Wang X, Sun J, Liu L, et al. Hypoxia regulates fibrosis-related genes via histone lactylation in the placentas of patients with preeclampsia. J Hypertens. 2022;40:1189–98.
Chen J, Qin P, Sun Y, Han S. Histone lactylation promotes cell proliferation, migration and invasion through targeting HMGB1 in endometriosis. J Biomed Res. 2023;37:470–8.
Wei L, Yang X, Wang J, Wang Z, Wang Q, Ding Y, et al. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J Neuroinflamm. 2023;20:208.
Lopez Krol A, Nehring HP, Krause FF, Wempe A, Raifer H, Nist A, et al. Lactate induces metabolic and epigenetic reprogramming of pro-inflammatory Th17 cells. EMBO Rep. 2022;23:e54685.
Gao M, Zhang N, Liang W. Systematic analysis of lysine lactylation in the plant fungal pathogen Botrytis cinerea. Front Microbiol. 2020;11:594743.
Luo Y, Yang Z, Yu Y, Zhang P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int J Biol Macromol. 2022;222:2225–43.
Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z, et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14:6523.
An S, Yao Y, Hu H, Wu J, Li J, Li L, et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023;14:457.
Sun T, Liu B, Li Y, Wu J, Cao Y, Yang S, et al. Oxamate enhances the efficacy of CAR-T therapy against glioblastoma via suppressing ectonucleotidases and CCR8 lactylation. J Exp Clin Cancer Res. 2023;42:253.
Jia M, Yue X, Sun W, Zhou Q, Chang C, Gong W, et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci Adv. 2023;9:eadg4993.
Miao Z, Zhao X, Liu X. Hypoxia induced β-catenin lactylation promotes the cell proliferation and stemness of colorectal cancer through the wnt signaling pathway. Exp Cell Res. 2023;422:113439.
Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X, et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022;39:110986.
Hong H, Chen X, Wang H, Gu X, Yuan Y, Zhang Z. Global profiling of protein lysine lactylation and potential target modified protein analysis in hepatocellular carcinoma. Proteomics. 2023;23:e2200432.
Jin J, Bai L, Wang D, Ding W, Cao Z, Yan P, et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 2023;24:e56052.
Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z, et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J Clin Invest. 2024;134:e174587.
Zong Z, Xie F, Wang S, Wu X, Zhang Z, Yang B. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell. 2024;187:2375–92.e33.
Mao Y, Zhang J, Zhou Q, He X, Zheng Z, Wei Y, et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024;34:13–30.
Fan M, Yang K, Wang X, Chen L, Gill PS, Ha T, et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci Adv. 2023;9:eadc9465.
Du S, Zhang X, Jia Y, Peng P, Kong Q, Jiang S, et al. Hepatocyte HSPA12A inhibits macrophage chemotaxis and activation to attenuate liver ischemia/reperfusion injury via suppressing glycolysis-mediated HMGB1 lactylation and secretion of hepatocytes. Theranostics. 2023;13:3856–71.
Martin TP, MacDonald EA, Elbassioni AAM, O’Toole D, Zaeri AAI, Nicklin SA. Preclinical models of myocardial infarction: from mechanism to translation. Br J Pharmacol. 2022;179:770–91.
Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72:865–7.
Wang K, Li Y, Qiang T, Chen J, Wang X. Role of epigenetic regulation in myocardial ischemia/reperfusion injury. Pharmacol Res. 2021;170:105743.
Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95.
Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87.
Koyama T, Munakata M, Akima T, Miyamoto K, Kanki H, Ishikawa S. Postconditioning with lactate-enriched blood for cardioprotection in ST-segment elevation myocardial infarction. J Vis Exp. 2019;147:e59672.
Xu GE, Yu P, Hu Y, Wan W, Shen K, Cui X, et al. Exercise training decreases lactylation and prevents myocardial ischemia-reperfusion injury by inhibiting YTHDF2. Basic Res Cardiol. 2024;119:651–71.
Yu W, Kong Q, Jiang S, Li Y, Wang Z, Mao Q, et al. HSPA12A maintains aerobic glycolytic homeostasis and Histone3 lactylation in cardiomyocytes to attenuate myocardial ischemia/reperfusion injury. JCI Insight. 2024;9:e169125.
Dossi CG, Vargas RG, Valenzuela R, Videla LA. Beneficial effects of natural compounds on experimental liver ischemia-reperfusion injury. Food Funct. 2021;12:3787–98.
Wang H, Xi Z, Deng L, Pan Y, He K, Xia Q. Macrophage polarization and liver ischemia-reperfusion injury. Int J Med Sci. 2021;18:1104–13.
Liu H, Fan J, Zhang W, Chen Q, Zhang Y, Wu Z. OTUD4 alleviates hepatic ischemia-reperfusion injury by suppressing the K63-linked ubiquitination of TRAF6. Biochem Biophys Res Commun. 2020;523:924–30.
Ding MJ, Fang HR, Zhang JK, Shi JH, Yu X, Wen PH, et al. E3 ubiquitin ligase ring finger protein 5 protects against hepatic ischemia reperfusion injury by mediating phosphoglycerate mutase family member 5 ubiquitination. Hepatology. 2022;76:94–111.
Li F, Zhang L, Xue H, Xuan J, Rong S, Wang K. SIRT1 alleviates hepatic ischemia-reperfusion injury via the miR-182-mediated XBP1/NLRP3 pathway. Mol Ther Nucleic Acids. 2020;23:1066–77.
Zhang S, Jiang S, Wang H, Di W, Deng C, Jin Z, et al. SIRT6 protects against hepatic ischemia/reperfusion injury by inhibiting apoptosis and autophagy related cell death. Free Radic Biol Med. 2018;115:18–30.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51.
Zhang J, Cao L, Gao A, Ren R, Yu L, Li Q, et al. E3 ligase RNF99 negatively regulates TLR-mediated inflammatory immune response via K48-linked ubiquitination of TAB2. Cell Death Differ. 2023;30:966–78.
Wei S, Gao Y, Dai X, Fu W, Cai S, Fang H, et al. SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. 2019;316:F20–31.
Tian Y, Russo RM, Li Y, Karmakar M, Liu B, Puskarich MA, et al. Serum citrullinated histone H3 concentrations differentiate patients with septic verses non-septic shock and correlate with disease severity. Infection. 2021;49:83–93.
García-Giménez JL, García-López E, Mena-Mollá S, Beltrán-García J, Osca-Verdegal R, Nacher-Sendra E, et al. Validation of circulating histone detection by mass spectrometry for early diagnosis, prognosis, and management of critically ill septic patients. J Transl Med. 2023;21:344.
Bakker J, Postelnicu R, Mukherjee V. Lactate: where are we now? Crit Care Clin. 2020;36:115–24.
Yuan Q, Yuan Y, Zheng Y, Sheng R, Liu L, Xie F, et al. Anti-cerebral ischemia reperfusion injury of polysaccharides: a review of the mechanisms. Biomed Pharmacother. 2021;137:111303.
Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci. 2021;23:14.
Zhang Q, Jia M, Wang Y, Wang Q, Wu J. Cell death mechanisms in cerebral ischemia-reperfusion injury. Neurochem Res. 2022;47:3525–42.
Hochrainer K. Protein modifications with ubiquitin as response to cerebral ischemia-reperfusion injury. Transl Stroke Res. 2018;9:157–73.
Aune SE, Herr DJ, Kutz CJ, Menick DR. Histone deacetylases exert class-specific roles in conditioning the brain and heart against acute ischemic injury. Front Neurol. 2015;6:145.
Fessler EB, Chibane FL, Wang Z, Chuang DM. Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery. Curr Pharm Des. 2013;19:5105–20.
Pickell Z, Williams AM, Alam HB, Hsu CH. Histone deacetylase inhibitors: a novel strategy for neuroprotection and cardioprotection following ischemia/reperfusion injury. J Am Heart Assoc. 2020;9:e016349.
Yao Y, Bade R, Li G, Zhang A, Zhao H, Fan L, et al. Global-scale profiling of differential expressed lysine-lactylated proteins in the cerebral endothelium of cerebral ischemia-reperfusion injury rats. Cell Mol Neurobiol. 2023;43:1989–2004.
Zhang L, Wang X, Che W, Zhou S, Feng Y. METTL3 silenced inhibited the ferroptosis development via regulating the TFRC levels in the Intracerebral hemorrhage progression. Brain Res. 2023;1811:148373.
He X, Wang Z, Ge Q, Sun S, Li R, Wang B. Lactylation of nuclear receptor coactivator 4 promotes ferritinophagy and glycolysis of neuronal cells after cerebral ischemic injury. Neuroreport. 2024;35:895–903.
Zhou J, Zhang L, Peng J, Zhang X, Zhang F, Wu Y, et al. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 2024;36:2054–68.e14.
Xiong XY, Pan XR, Luo XX, Wang YF, Zhang XX, Yang SH, et al. Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation. Theranostics. 2024;14:4297–317.
Wang L, Cai Z, Gu Q, Xu C. cGAS deficiency regulates the phenotypic polarization and glycolysis of microglia through lactylation in hypoxic-ischemic encephalopathy cell model. Biochem Genet. 2024;62:3961–76.
Song C, Fang X, Fang N, Hu F. Buyang Huanwu Decoction suppresses ischemic stroke by suppressing glycolysis and cell apoptosis in rat brain microvascular endothelial cells. Brain Res Bull. 2024;215:111032.
Zhang W, Xu L, Yu Z, Zhang M, Liu J, Zhou J. Inhibition of the glycolysis prevents the cerebral infarction progression through decreasing the lactylation levels of LCP1. Mol Biotechnol. 2023;65:1336–45.
Wang M, He T, Meng D, Lv W, Ye J, Cheng L, et al. BZW2 modulates lung adenocarcinoma progression through glycolysis-mediated IDH3G lactylation modification. J Proteome Res. 2023;22:3854–65.
Zhao Y, Zhang M, Huang X, Liu J, Sun Y, Zhang F, et al. Lactate modulates zygotic genome activation through H3K18 lactylation rather than H3K27 acetylation. Cell Mol Life Sci. 2024;81:298.
Wang X, Fan W, Li N, Ma Y, Yao M, Wang G, et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol. 2023;24:87.
Yu Y, Huang X, Liang C, Zhang P. Evodiamine impairs HIF1A histone lactylation to inhibit Sema3A-mediated angiogenesis and PD-L1 by inducing ferroptosis in prostate cancer. Eur J Pharmacol. 2023;957:176007.
Xu K, Zhang K, Wang Y, Gu Y. Comprehensive review of histone lactylation: structure, function, and therapeutic targets. Biochem Pharmacol. 2024;225:116331.
Raychaudhuri D, Singh P, Hennessey M, Chakraborty B, Tannir AJ, Trujillo-Ocampo A, et al. Histone lactylation drives CD8+ T cell metabolism and function. Nat Immunol. 2024;25:2140–51.
Huang Y, Wang C, Zhou T, Xie F, Liu Z, Xu H, et al. Lumican promotes calcific aortic valve disease through H3 histone lactylation. Eur Heart J. 2024;45:3871–85.
Li G, Wang D, Zhai Y, Pan C, Zhang J, Wang C, et al. Glycometabolic reprogramming-induced XRCC1 lactylation confers therapeutic resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab. 2024;36:1696–710.e10.
Xia J, Qiao Z, Hao X, Zhang Y. LDHA-induced histone lactylation mediates the development of osteoarthritis through regulating the transcription activity of TPI1 gene. Autoimmunity. 2024;57:2384889.
Hong W, Zeng X, Wang H, Tan X, Tian Y, Hu H, et al. PGC-1α loss promotes mitochondrial protein lactylation in acetaminophen-induced liver injury via the LDHB-lactate axis. Pharmacol Res. 2024;205:107228.
Fan W, Zeng S, Wang X, Wang G, Liao D, Li R, et al. A feedback loop driven by H3K9 lactylation and HDAC2 in endothelial cells regulates VEGF-induced angiogenesis. Genome Biol. 2024;25:165.
Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021; https://doi.org/10.1136/gutjnl-2020-323263.
Hagihara H, Shoji H, Otabi H, Toyoda A, Katoh K, Namihira M, et al. Protein lactylation induced by neural excitation. Cell Rep. 2021;37:109820.
Pandkar MR, Sinha S, Samaiya A, Shukla S. Oncometabolite lactate enhances breast cancer progression by orchestrating histone lactylation-dependent c-Myc expression. Transl Oncol. 2023;37:101758.
Longhitano L, Giallongo S, Orlando L, Broggi G, Longo A, Russo A, et al. Lactate rewrites the metabolic reprogramming of uveal melanoma cells and induces quiescence Phenotype. Int J Mol Sci. 2022;24:24.
Wang ZH, Zhang P, Peng WB, Ye LL, Xiang X, Wei XS, et al. Altered phenotypic and metabolic characteristics of FOXP3+CD3+CD56+ natural killer T (NKT)-like cells in human malignant pleural effusion. Oncoimmunology. 2022;12:2160558.
Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2020;2:882–92.
Sun S, Xu X, Liang L, Wang X, Bai X, Zhu L, et al. Lactic acid-producing probiotic Saccharomyces cerevisiae attenuates ulcerative colitis via suppressing macrophage pyroptosis and modulating gut microbiota. Front Immunol. 2021;12:777665.
Wang P, Xie D, Xiao T, Cheng C, Wang D, Sun J, et al. H3K18 lactylation promotes the progression of arsenite-related idiopathic pulmonary fibrosis via YTHDF1/m6A/NREP. J Hazard Mater. 2024;461:132582.
Han H, Zhao Y, Du J, Wang S, Yang X, Li W, et al. Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun Ageing. 2023;20:63.
Ma W, Ao S, Zhou J, Li J, Liang X, Yang X, et al. Methylsulfonylmethane protects against lethal dose MRSA-induced sepsis through promoting M2 macrophage polarization. Mol Immunol. 2022;146:69–77.
Chaudagar K, Hieromnimon HM, Kelley A, Labadie B, Shafran J, Rameshbabu S, et al. Suppression of tumor cell lactate-generating signaling pathways eradicates murine PTEN/p53-deficient aggressive-variant prostate cancer via macrophage phagocytosis. Clin Cancer Res. 2023;29:4930–40.
Rho H, Terry AR, Chronis C, Hay N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023;35:1406–23.e8.
He Y, Ji Z, Gong Y, Fan L, Xu P, Chen X, et al. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 2023;42:112033.
Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L, et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. 2024;20:114–30.
Xie B, Lin J, Chen X, Zhou X, Zhang Y, Fan M, et al. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol Cancer. 2023;22:151.
Chaudagar K, Hieromnimon HM, Khurana R, Labadie B, Hirz T, Mei S, et al. Reversal of lactate and PD-1-mediated macrophage immunosuppression controls growth of PTEN/p53-deficient prostate cancer. Clin Cancer Res. 2023;29:1952–68.
Wu J, Hu M, Jiang H, Ma J, Xie C, Zhang Z, et al. Endothelial cell-derived lactate triggers bone mesenchymal stem cell histone lactylation to attenuate osteoporosis. Adv Sci. 2023;10:e2301300.
Zhou J, Xu W, Wu Y, Wang M, Zhang N, Wang L, et al. GPR37 promotes colorectal cancer liver metastases by enhancing the glycolysis and histone lactylation via Hippo pathway. Oncogene. 2023;42:3319–30.
Huang ZW, Zhang XN, Zhang L, Liu LL, Zhang JW, Sun YX, et al. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct Target Ther. 2023;8:391.
Chen J, Zhang M, Liu Y, Zhao S, Wang Y, Wang M, et al. Histone lactylation driven by mROS-mediated glycolytic shift promotes hypoxic pulmonary hypertension. J Mol Cell Biol. 2023;14:mjac073.
Yin D, Jiang N, Cheng C, Sang X, Feng Y, Chen R, et al. Protein lactylation and metabolic regulation of the zoonotic parasite Toxoplasma gondii. Genomics Proteomics Bioinformatics. 2023;21:1163–81.
Yang W, Wang P, Cao P, Wang S, Yang Y, Su H, et al. Hypoxic in vitro culture reduces histone lactylation and impairs pre-implantation embryonic development in mice. Epigenetics Chromatin. 2021;14:57.
Wang J, Liu Z, Xu Y, Wang Y, Wang F, Zhang Q, et al. Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front Cell Infect Microbiol. 2022;12:913815.
Wang X, Ying T, Yuan J, Wang Y, Su X, Chen S, et al. BRAFV600E restructures cellular lactylation to promote anaplastic thyroid cancer proliferation. Endocr Relat Cancer. 2023;30:e220344.
Merrick CJ. Histone lactylation: a new epigenetic axis for host-parasite signalling in malaria? Trends Parasitol. 2023;39:12–16.
Dai W, Wu G, Liu K, Chen Q, Tao J, Liu H, et al. Lactate promotes myogenesis via activating H3K9 lactylation-dependent up-regulation of Neu2 expression. J Cachexia Sarcopenia Muscle. 2023;14:2851–65.
Zhao W, Wang Y, Liu J, Yang Q, Zhang S, Hu X, et al. Progesterone activates the histone lactylation-Hif1α-glycolysis feedback loop to promote decidualization. Endocrinology. 2023;165:bqad169.
Wang L, Wang Y, Meng M, Ma N, Wei G, Huo R, et al. High-concentrate diet elevates histone lactylation mediated by p300/CBP through the upregulation of lactic acid and induces an inflammatory response in mammary gland of dairy cows. Microb Pathog. 2023;180:106135.
Xie J, Hong S, Zhang X, Li Y, Xie R. Inhibition of glycolysis prevents behavioural changes in mice with MK801-induced SCZ model by alleviating lactate accumulation and lactylation. Brain Res. 2023;1812:148409.
Yin X, Li M, Wang Y, Zhao G, Yang T, Zhang Y, et al. Herbal medicine formula Huazhuo Tiaozhi granule ameliorates dyslipidaemia via regulating histone lactylation and miR-155-5p biogenesis. Clin Epigenetics. 2023;15:175.
Xu H, Li L, Wang S, Wang Z, Qu L, Wang C, et al. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine. 2023;118:154940.
Lin J, Ji Z, Di Z, Zhang Y, Yan C, Zeng S. Overexpression of Tfap2a in mouse oocytes impaired spindle and chromosome organization. Int J Mol Sci. 2022;23:14376.
Duan W, Liu W, Xia S, Zhou Y, Tang M, Xu M, et al. Warburg effect enhanced by AKR1B10 promotes acquired resistance to pemetrexed in lung cancer-derived brain metastasis. J Transl Med. 2023;21:547.
Zheng P, Mao Z, Luo M, Zhou L, Wang L, Liu H, et al. Comprehensive bioinformatics analysis of the solute carrier family and preliminary exploration of SLC25A29 in lung adenocarcinoma. Cancer Cell Int. 2023;23:222.
Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao H, et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell. 2024;187:294–311.e21.
Sun W, Jia M, Feng Y, Cheng X. Lactate is a bridge linking glycolysis and autophagy through lactylation. Autophagy. 2023;19:3240–1.
Sun S, Xu Z, He L, Shen Y, Yan Y, Lv X, et al. Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed α-tubulin lactylation. Nat Commun. 2024;15:8377.
Chen X, Huang W, Zhang J, Li Y, Xing Z, Guo L, et al. High-intensity interval training induces lactylation of fatty acid synthase to inhibit lipid synthesis. BMC Biol. 2023;21:196.
Wang Y, Chen L, Zhang M, Li X, Yang X, Huang T, et al. Exercise-induced endothelial Mecp2 lactylation suppresses atherosclerosis via the Ereg/MAPK signalling pathway. Atherosclerosis. 2023;375:45–58.
Yao G, Yang Z. Glypican-3 knockdown inhibits the cell growth, stemness, and glycolysis development of hepatocellular carcinoma cells under hypoxic microenvironment through lactylation. Arch Physiol Biochem. 2024;130:546–54.
Liao J, Chen Z, Chang R, Yuan T, Li G, Zhu C, et al. CENPA functions as a transcriptional regulator to promote hepatocellular carcinoma progression via cooperating with YY1. Int J Biol Sci. 2023;19:5218–32.
Xu X, Zhang DD, Kong P, Gao YK, Huang XF, Song Y, et al. Sox10 escalates vascular inflammation by mediating vascular smooth muscle cell transdifferentiation and pyroptosis in neointimal hyperplasia. Cell Rep. 2023;42:112869.
Acknowledgements
Figures in this review were created using Figdraw.com.
Funding
This research was supported by grants from the National Natural Science Foundation of China (No. 81873930), Sichuan Science and Technology Program (MZGC20240086), Luzhou Science and Technology Program (No. 2023SYF099).
Author information
Authors and Affiliations
Contributions
FW and JZ performed study concept and design; FW and GM generated the data and assembled the figures and tables; FW, GM, ZY, ZS, XL, XF, YC, and JZ performed writing, review and revision of the paper; YC and JZ performed project administration and funding acquisition. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Fx., Mu, G., Yu, Zh. et al. Lactylation: a promising therapeutic target in ischemia-reperfusion injury management. Cell Death Discov. 11, 100 (2025). https://doi.org/10.1038/s41420-025-02381-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-025-02381-4
This article is cited by
-
Lactate regulates the YTHDF2-FTH1 axis to promote cardiomyocyte ferroptosis and aggravate myocardial ischemia-reperfusion injury
Scientific Reports (2026)
-
Prognostic role of creatine kinase–MB in elderly patients with sepsis
European Journal of Medical Research (2025)
-
Roles of lactylation in lipid metabolism and related diseases
Cell Death Discovery (2025)
-
MicroRNA-451: A multifaceted regulator in cerebral and cardiac ischemia-reperfusion injury - from molecular mechanisms to therapeutic potential
Molecular Biology Reports (2025)