Abstract
A hallmark of cancer biology is resistance to apoptosis. BCL-2 is an anti-apoptotic molecule that is being overexpressed in several myeloid diseases, such as acute myeloid leukemia and myelodysplastic syndromes, but also in several lymphoid cancers, such as acute lymphoblastic leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphomas and multiple myeloma. Venetoclax (VEN) is a BCL-2 small molecule inhibitor. Data about its structure, biochemical characteristics and in vitro efficacy against several blood cancer cell lines were first reported in 2013. Shortly after, the first clinical trials reported that single-agent VEN provides no long-term survival benefits. In contrast, when used in combination, VEN led to significantly improved outcomes and eventually to its first US FDA approvals in 2018. As the modern approach to treating hematological malignancies are the chemotherapy-free regimen, in the current manuscript, we provide a comprehensive view on all available therapies that are considered to be chemotherapy-free, with a special emphasis on acute myeloid leukemia (AML), where phase I-III clinical trials have provided the most data.
Similar content being viewed by others
Facts
-
Venetoclax brought a major breakthrough in the clinical management of AML
-
Chemotherapy-free regimens are associated with better patient reported outcomes
-
Venetoclax alone does not always provide long-term survival benefits, but in association with immune therapies and/or targeted therapies, the outcomes are far better than current standard-of-care
Open questions
-
Will chemotherapy-free regiments completely replace current chemotherapy-based regimens?
-
Will venetoclax alone or in combination with immune therapies and/or targeted therapies represent the backbone of future chemotherapy-free alternatives?
Introduction
A hallmark of cancer biology is resistance to apoptosis [1]. BCL-2 is an antiapoptotic molecule that is being overexpressed in several myeloid diseases such as acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), but also in several lymphoid cancers such as acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphomas, and multiple myeloma (MM) [2,3,4,5,6]. AML is a blood cancer characterized by the clonal proliferation of immature myeloid precursors, leading to impaired hematopoiesis in the bone marrow [7]. Evaluation of the performance status of the patient and a thorough morphologic, immunophenotypic, and molecular analysis are essential to classify and risk-stratify AML patients in order to design individualized treatment plans [8]. While the “7 + 3” cytarabine-anthracycline-based intensive chemotherapy (IC) regimen has long been considered the gold standard, advances in genomic profiling have transformed AML treatment with the introduction of multiple targeted therapies. FLT3-inhibitors like midostaurin or gilteritinib and IDH1- or IDH2-inhibitors, such as ivosidenib and enasidenib are already FDA-approved agents integrated in the daily practice. Additionally, gemtuzumab ozogamicin (GO) is a feasible option for patients with CD33-positive AML, especially in favorable-risk cases and also as an effective cytoreductive agent [9, 10]. Venetoclax (VEN) is a BCL-2 small-molecule inhibitor. Data about its structure, biochemical characteristics and in vitro efficacy against several blood cancer cell lines have been first published in 2013 [6]. Shortly after, the first clinical trials reported that single-agent VEN provided no long-term survival benefits [11]. In contrast, when used in combination, VEN led to significantly improved outcomes and trials leading to its US FDA approval, starting from 2018, have been summarized in Table 1. Tables 2–4 outline relevant clinical trials of VEN-based, chemo-free combinations which hold the potential to transform the therapeutic landscape for AML in the future. All chemotherapy-containing regimens were excluded from our analysis. Our review provides a thorough and visionary overview of current clinical status, unknowns and drawbacks of VEN therapy in AML. We summarize immunotherapy and targeted therapy combinations to provide state-of-the-art clinical insights for clinicians and highlight important gaps in current knowledge regarding several treatment aspects, such as which combination is best for specific patient subgroups, the optimal number of cycles, minimal residual disease (MRD)-tailored decisions, and the optimal timing for treatment discontinuation.
Hypomethylating agents (HMA)
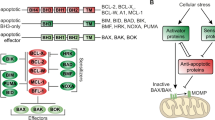
The combination of HMA, such as azacitidine (AZA) and decitabine (DEC), with VEN has been shown to work synergistically, their mechanism of action being described in Fig. 1.
A The methylation as an epigenetic process in the DNA, leading to methyl cytosine (5-mC), is used as regulators of different promoters and protein-encoding genes. B Cytosine analogs— azacytidine (Aza) and decitabine (Dec)—mimic cytosine but have an extra nitrogen instead of a carbon in the 5’ position. During cell division, the analogs act as demethylating agents, replacing wild-type cytosine, thus the methyl group cannot be incorporated into DNA, leading to global demethylation. C The overview of the mechanism of action of venetoclax, binding to BCL-2 and letting BH3-peptides free to bind to BAK/BAX, triggering apoptosis. D The expected outcome of a combination therapy using venetoclax and HMA, with hypomethylated tumor cells and high apoptosis rates.
The first question that may arise when combining HMA with VEN is whether efficacy is maintained across all age groups. The phase 2 M14-358 (NCT02203773) and phase 3 VIALE-A (NCT02993523) studies were the first to demonstrate that 7 days of AZA or 5 days of DEC combined with 28 days of VEN significantly outperform AZA + placebo in older adults (≥75 years) with newly-diagnosed (N/D) AML, particularly those unfit for intensive chemotherapy [12, 13]. A recent pooled analysis of VIALE-A demonstrated consistent safety and efficacy data across all elderly cohorts (75–79 years, 80–84 years, ≥85 years) [14]. A recent update also indicated a survival advantage in younger (<75 years) patients with IDH1-, IDH2-, or NPM1-mutant AML. This trend, however, has not been observed in the case of FLT3- or TP53 mutations [15]. Younger, adverse-risk AML patients, including those with complex karyotypes or TP53-mutations achieved, however, higher rates of response with 5 days of DEC + VEN compared to AZA + VEN based on the results of the phase 2 NCT04752527 [16]. Thus, genetic subtypes, rather than age, are more powerful predictors of response and outcomes. IDH1-mutated AML treated with HMA + VEN compared favorable to IDH2-mutations, with the mention that the small sample size and the enrichment for NPM1-mutations in the IDH1 cohort may have been confounding factors [17]. ASXL1 mutations without the presence of adverse karyotype or TP53 mutations also predicted better responses in N/D patients [18]. In contrast, a novel survival prediction model indicated a 1-year survival <1% in patients with ASXL1, RAS or TP53 mutations that failed frontline HMA + VEN vs 42% in the absence of these [19]. N/KRAS, KMT2A, and SF3B1 mutations were also associated with worse outcomes [20].
A second important clinical question is whether longer treatment duration might improve the depth and duration of response. Beyond genetic risk factors, monocytic differentiation identified by morphology or flow cytometry has also been associated with resistance to HMA + VEN (CR rates of 26.7% in monocytic-like vs 80% in non-monocytic-like subtypes, P < 0.001) [21]. A novel approach of 10-day DEC instead of the classical 5-day regimen, however, improved response rates of monocytic leukemia, reaching an overall response rate (ORR) of 86% with 68% of these being sustained at 1 year. Also, substantial improvements in response rates have been observed with 10-day DEC + VEN for RUNX1, FLT3, and TP53 mutations with an impressive ORR for the overall population of 89% and median OS of 18.1 months (95% CI, 10—not reached) [22]. Longer, 10-day administration of DEC was well tolerated in older subjects, without any therapy-related mortality and a 1-year progression-free survival (PFS) of 84% [17].
Randomized, controlled, trials comparing IC with HMA + VEN in N/D AML are lacking. The phase 2 NCT03573024 concluded that IC and AZA + VEN in non-favorable risk, young (<60 years) AML patients led to comparable ORR (50 vs 61%). However, a lower median number of days admitted to the hospital (30 vs 7.5, P < 0.0001), lower median units of platelet (11 vs 4, P = 0.0076) or red blood cell transfusions (9 vs 3.5, P = 0.0068) and a lower incidence of infections (93 vs 39%, P = 0.0071) favored AZA + VEN [23]. The complex morphologic, genetic, and molecular subtypes of AML make the selection of an ideal treatment and the comparison of IC with lower intensity regimens extremely difficult. The VINCENT phase 2 trial is currently enrolling N/D, NPM1-mutated, FLT3-wild-type subjects to assess AZA + VEN vs 7 + 3 + GO [24]. A retrospective study that examined younger (60–75 years) AML patients treated with HMA + VEN did not show any difference in CR rates and median overall survival (OS), in case of NPM1, TP53, and FLT3-ITD mutant AML compared to IC [25]. Similar outcomes have been reported in N/D AML patients (age ≥60 years) carrying an IDH1- or IDH2-mutation [26]. Higher ORR with AZA + VEN compared to IC, however, have reported with mutations in RUNX1 (82% vs 45%, P = 0.003), chromatin-cohesin genes (ASXL1, EZH2, BCOR, STAG2) (77 vs 47%, P = 0.005) or myelodysplasia-related genes (86 vs 43%, P = 0.001), with higher overall MRD-negative CR rates in the AZA + VEN arm (46 vs 19%, P < 0.001) [27]. A potential benefit of IC over HMA + VEN was linked to favorable-risk AML, normal cytogenetics and RAS pathway mutations [25]. Patients aged 60–75 years with very-high risk cytogenetics, such as inv(3)(q21.3q26.2) t(3;3)(q21.3;q26.2)/GATA2:MECOM(EVI1), t(3q26.2;v)/MECOM(EVI1)-rearranged, complex karyotype or monosomal karyotype, but without a TP53 mutation, achieved longer median OS when treated with IC (14 months, 95% CI 10-30), compared to AZA + VEN (8.0 months, 95% CI 5.2-15) [28]. Monosomy 5 or deletions in chromosomes 5 or 7 have also been linked to a longer median OS when treated with IC vs AZA + VEN. However, when adjusted by age to the 60–75 age range, there was no statistically significant difference in survival [29]. In case of previously untreated secondary AML, AZA + VEN seems to be superior to the “7 + 3” regimen, but there was no difference in efficacy compared to CPX-351 [30]. The myeloMATCH phase 2 trial (NCT05554406), among others, is currently recruiting young (<60 years), adverse risk N/D AML patients to directly compare two cycles of induction with AZA + VEN vs liposomal “7 + 3” vs liposomal “7 + 3” + VEN vs liposomal cytarabine + standard daunorubicin [31]. Also, the efficacy of HMA + VEN in secondary AML patients with prior exposure to hypomethylating agents is currently under investigation in the phase 2 NCT04905810 trial. In case of DEC + VEN, the first interim results of the NCT05177731 phase 2 trial comparing it to standard “7 + 3” in young adults (<60 years) with N/D AML have also been published. DEC + VEN showed significantly higher complete response (CR) and MRD-negativity rates with a significantly decreased incidence of grade 3 adverse events in the intermediate- and adverse-risk AML group, especially for patients aged 40 and older (P < 0.001) [32].
Apart from the selection of an optimal regimen, the length of VEN-based induction therapies is an ongoing topic of discussion. Table 5 summarizes the results of several studies comparing the length of VEN therapy in N/D AML patients ineligible for IC. Karrar et al. reported that patients with one or more favorable-risk mutations (NPM1, IDH2, and DDX41) and without any adverse-risk features (TP53, FLT-ITD, and RUNX1) showed no significant difference in CR rates with 14 days vs 21 days vs 28 days of VEN exposure (92, 90, and 100%; P = 0.19). Similarly, in patients with unfavorable cytogenetics, CR rates were not substantially improved in those who received VEN for 28 days (44%) compared to 21 days (60%) or 14 days (22%) (P = 0.18). CR rates of TP53-mutated patients were also comparable (P = 0.81). Moreover, duration of response was similar in patients receiving VEN for 14 days (8.7 months, 95% CI 1–37) vs 28 days (8.3 months, 95% CI 1–5, P = 0.12) [33]. These shorter cycles might especially favor the outcomes of the older individuals.
A retrospective study with only 14 days of VEN showed improved PFS, longer OS and less toxicity than administration for ≥15-day [34]. Another retrospective analysis showed that patients with ≤14-day VEN exposure had significantly improved median PFS (15.8 months vs 8.7 months, P = 0.01) and OS (24.7 months vs 11.3 months, P = 0.006) compared to the ≥15-day arm. Also, a reduced median duration of VEN/cycle was associated with a significant decrease in risk of death (HR 0.18, 95% CI 0.07–0.48, P = 0.0007). Moreover, extended cycle intervals (≥35 days), to allow count recovery, were not linked to an increased risk of death (HR 0.59, 95% CI 0.27–1.26; P = 0.17). A significant survival benefit of reduced treatment duration has been observed in TP53-mutated AML (median OS 14.7 months vs 8.7 months; P = 0.026) [35]. Cui et al. confirmed that 14-day administration is comparable to outcomes of VIALE-A, and it is the first study to report that the FAB-M5 AML patients achieved a superior outcome with 14-day VEN compared to other subtypes [36]. In contrast, Ginosyan et al. reported that 21 days of VEN led to longer OS (6.1 months vs 17.6 months, HR 3.08, 95% CI, 1.10–8.59, P = 0.032) and lower relapse rates (HR 6.56, 95%, CI 2.37–18.2, P < 0.001) compared to the 14-day cohort [37]. Since results are controversial, more prospective, randomized trials are required before drawing any conclusions. The Beat AML phase 2 trial (NCT03013998), for instance, is currently recruiting patients to directly compare 14 days vs 28 days of VEN.
The most recent retrospective study conducted by Willekens et al. also compared 7-days VEN vs SOC VEN regimens (14 days or more) and found no difference in ORR, CR rate and MRD-negativity rate. The patients, however, tolerated more cycles (median of 6 vs 3, P < 0.01) with a lower median time between cycles (28–30 days vs 36–39 days). In terms of toxicity, early 8-week mortality was significantly decreased (6 vs 16%, P = 0.03) with a reduction in the need for platelet transfusions (62 vs 72%, P = 0.02). A prospective, phase 3 trial (SEVENAZA) has been initiated in order to compare efficacy and outcomes of the “7 + 7” VEN + AZA regimen [38]. As a conclusion, multiple factors might play a role in improving outcomes with the reduction of VEN treatment duration, such as reduced mortality or improved tolerance leading to a lower discontinuation rate, a lower rate of dose reductions and thus an increased HMA exposure. Also, a reduction of continuous BCL-2 inhibition might decrease intracellular adaptive mechanisms that may lead to drug resistance [39].
Another clinical trend is to reduce not just the dose of VEN, but also the exposure to HMA. The phase 2 VENAZA-5S (NCT05833438) trial is currently recruiting subjects to assess the safety and efficacy of a reduced 5-day AZA schedule in elderly, frail patients. Another study, NCT06073730, assessed the efficacy of a 3-day DEC and 14-day VEN induction followed by 2-day decitabine consolidation cycles in elderly patients unfit for IC. Preliminary data on 47 subjects suggests that 3-day DEC induction leads to superior outcomes and significantly less myelotoxicity than previously reported with 5-day or 10-day administration [40]. The phase 2 NCT06285136 trial investigated the same regimen but in younger patients (<65 years). Results seem really promising with 18 out of 23 patients reaching MRD-negativity [41]. NCT05184842 investigated 28-day VEN with low-dose decitabine (0.2 mg/kg weekly vs 20 mg/m2/day for 5 days). In this trial, the CR rates (61 vs 68%) and median OS (16.1 months vs 14.7 months) were comparable to those observed in VIALE-A [42].
MRD assessment is crucial for the evaluation of treatment responses and the prediction of relapse. Monitoring MRD allows for early intervention, MRD-guided decision-making is still limited to clinical trial settings. We identified several studies that assessed the prognostic value of different MRD measurement tools, optimal MRD threshold cut-offs or MRD-guided treatment discontinuation of HMA + VEN combinations. Importantly, MRD conversion in earlier HMA + VEN treatment cycles did not improve outcomes. MRD-negativity with a threshold of 10−3, regardless of the timepoint of conversion, however, has been a favorable prognostic factor, achieving OS of 34.2 months (95% CI, 27.7–44.0) vs 18.7 months (95% CI, 12.9–23.5) [15, 43]. MRD negativity after HMA + VEN in NPM1-mutations, determined by digital droplet PCR, did not impact OS or PFS. MRD negativity by flow, however, was predictive of better survival [44]. A retrospective study confirmed the efficacy of AZA + VEN reinduction in case of post-IC MRD, 19/27 of treated patients achieving MRD-negativity. NPM1 or FLT3-ITD mutations and an early MRD relapse (less than 12 months after CR) were not associated with subsequent MRD response failure following AZA + VEN [45]. Patients not reaching CR after one cycle of IC, however, seem to benefit more by a second cycle of IC than switching to HMA + VEN [46]. In case of relapse in the first 6 months after achievement of CR, the survival benefit of IC was not significant anymore, median OS being comparable (9 months for IC vs 6 months for HMA + VEN, p = 0.4). Age, ELN risk stratification, and cytogenetics did not significantly impact survival either [46]. MRD-guided discontinuation of AZA has also been tested in the phase 2 NCT03466294, without any benefits. Moreover, after azacitidine was stopped, its reintroduction in case of relapse could not bring a new response, or a decrease in MRD levels [44]. Thus, HMA + VEN maintenance therapy cycles seem to be crucial for durable responses. It is currently unknown whether decreasing HMA doses or lowering/increasing the recommended number of cycles is better to optimize the efficacy of the incidence of adverse events. Recent data suggests that lower doses of subcutaneous (SC) AZA (50 mg/m2) maintain efficacy while decreasing long-term toxicities. Patients with NPM1-, IDH1-, or IDH2-mutations showed a 2-year PFS of 79% (95% CI, 60–100) with no relapses occurring in the first year of maintenance. Mutations in FLT3, RAS, TET2, or DNMT3A were not significantly associated with differences in PFS [41]. The number of cycles in the clinical trials summarized by us ranges from six to 24. Most of these have not yet published any results.
Outpatient induction or maintenance with IV/SC HMA + VEN in the NCT03941964 trial reported that adverse events were consistent with those seen in inpatient settings and there were no safety concerns [47]. To facilitate outpatient administration, improve patient quality of life, and improve safety, all-oral HMA + VEN combinations have also been designed. CC-486, an oral formulation of AZA given for 14 days/cycle demonstrated comparable response rates with conventional IV or SC AZA in R/R AML patients in the phase 1 OMNIVERSE (NCT04887857) and NCT05287568 trials [48, 49]. CC-486 + VEN vs CC-486 + placebo as maintenance therapy for patients 18 years and older with N/D AML in first CR after IC is also tested in the phase III randomized, double-blind VIALE-M (M19-708) study (NCT04102020). ASTX727, oral DEC, +VEN is another all-oral option, both for N/D and R/R AML patients. The phase 1 NCT04657081 was the first trial to prove the safety of ASTX727 + VEN in older patients unfit for IC [50]. NCT04746235 is a phase 2 trial that confirmed the efficacy of this combination in elderly patients, with a median OS of 11.5 months (95% CI 9.1–16.6) in the frontline and 7.2 months (95% CI 6.3–NA) in the salvage therapy cohort. There was no significant difference in OS in younger patients compared to those aged 80 years or more (P = 0.825). Also, the same study demonstrated that the European Leukemia Net (ELN) risk classification published in 2022 did not effectively stratify patients in terms of OS (P = 0.84) [51]. Genetic groups, however, successfully predicted outcomes, TP53, N/KRAS and FLT3-ITD mutations being associated with a shorter duration of response [52]. The phase 3 ASCERTAIN (NCT03306264) study directly compared the pharmacokinetics and efficacy of ASTX727 vs IV DEC and found no significant difference in plasma concentrations and efficacy [53]. Efficacy of all-oral 5-day ASTX727 + 21-day VEN induction is directly compared with the standard “7 + 3” regimen in younger, fit patients in the phase 2 NCT04817241 trial. NCT04975919 enrolled R/R AML patients and administered 10 days of ASTX727 + 21 or 28 days of VEN/cycle and achieved a median OS of 9.1 months and CR in four of seven VEN-naive and 1 of 11 VEN-exposed patients suggesting that patients without VEN-based initial induction regimens may benefit more from this combination [54]. NCT05010772 demonstrated that the addition of VEN, gilteritinib or ivosidenib to ASTX727 is a feasible all-oral maintenance therapy approach [55].
Even in the era of targeted therapies, transplantation in eligible patients remains an essential factor in improving long-term prognosis. Whether VEN-based regimens are optimal as bridging therapies to transplant remains a critical clinical question. The phase 2 VEN-DEC GITMO (NCT04476199) demonstrated high efficacy and safety of two cycles of 5-day DEC + VEN as bridging to allotransplant [56]. A longer, 10-day DEC + VEN combination also proved to be an active salvage and bridging therapy in molecularly predefined R/R AML subpopulations, particularly in the case of NPM1, IDH1, IDH2, and FLT3-mutations [22]. The phase 2 GIMEMA AML2521 (NCT04867928) study enrolled NPM1-mutated MRD-positive AML patients following two cycles of IC. AZA + VEN led to the achievement of a molecular response in 80% of these patients. Therefore, this approach might be a feasible bridging therapy for transplant in the future [57]. The phase 2 ERASE study (NCT05554419) is currently enrolling patients to directly compare the efficacy in terms of MRD-conversion before undergoing allotransplantation of cytarabine alone vs cytarabine + VEN vs cytarabine + daunorubicin vs AZA + VEN. AZA + VEN proved to be safe and active in post-transplant R/R disease as well, with 57% (30/52) of patients reaching CR and 18.3% (9/52) achieving MRD-negativity [58]. Post-allotransplant maintenance with all-oral HMA + VEN is also currently being tested in NCT05799079.
FLT3-inhibitors
Current guidelines do not recommend the administration of frontline FLT3-inhibitors in IC-ineligible patients. We identified no trial assessing the efficacy of midostaurin, a first-generation FLT3-inhibitor, with chemo-free VEN-based regimens. However, case series report efficacy of 21-day/cycle midostaurin + VEN in t(8;21)(q22;q22.1)/AML1-ETO) core binding factor-AML associated with KIT mutations, R/R to frontline HMA + VEN regimens [59].
Although no survival benefit of the second-generation FLT3-inhibitor gilteritinib + AZA combination has been shown in the NCT02752035 trial, improved efficacy was reported in NCT03625505 with gilteritinib + VEN in the case of R/R AML [60, 61]. Later, the NCT04140487 trial added AZA to the gilteritinib + VEN backbone which demonstrated modest survival in R/R patients, but significantly increased efficacy in N/D patients unfit for IC, with 25 of 27, reaching MRD-negativity by a FLT3, polymerase chain reaction (PCR)-based assay. However, OS and median PFS have not yet been reached and significant toxicities have been reported [62]. A retrospective analysis of the triplet in N/D patients reported a median OS of 28.1 months, outcomes being superior in case of non-FLT3-ITD mutations (median OS of 39.1 months vs 24.5 months). NRAS, KRAS, PTPN11, CBL, NF1, and BRAF mutations have been associated with relapses. Outcomes have not been influenced by age groups (≥75 years vs <75 years, P = 0.65) [63]. The phase 2 NCT05520567 trial is currently enrolling elderly N/D patients to confirm the efficacy of the triplet therapy in patients 75 or older. Beat AML S8 (NCT03013998) assessed gilteritinib with DEC + VEN, and the preliminary data showed high efficacy, with tolerable adverse events and no treatment-related mortality [64]. Also, an all-oral triplet therapy with ASTX727 proved to be safe and showed an ORR of 53% in R/R patients [65]. NCT05010122 is currently recruiting N/D patients to assess this all-oral combination. Therefore, currently available data suggest that the addition of 80 mg gilteritinib daily to VEN alone or AZA/DEC + VEN to earlier treatment phases leads to superior outcomes in FLT3-mutated AML patients. Optimal treatment duration with gilteritinib remains to be determined. The MyeloMATCH MM1OA-EA02 substudy (NCT06317649) is directly comparing 28-day vs 14-day administration of gilteritinib [66].
Another active second-generation FLT3-inhibitor tested with AZA + VEN or DEC + VEN is quizartinib. Preliminary data from the phase 1/2 VEN-A-QUI trial indicated comparable efficacy to gilteritinib-containing triplets in N/D patients regardless of their FLT3 mutational status. However, early toxicity and a case of early death have been observed, warranting further evaluation of the safety of this combination [67]. While FLT3-ITD mutations led to worse outcomes with gilteritinib-containing triplets, preliminary data from the phase 2 NCT03661307 suggest the promising efficacy of quizartinib + DEC + VEN combination, with all five N/D patients achieving CR, with 4 of 5 achieving MRD-negativity by FLT3 PCR and 2 of 5 by flow cytometry [68]. The quizartinib-containing triplet has also been shown to have high activity in R/R patients with prior exposure to FLT3-inhibitors (78% previously treated with gilteritinib) [69]. Future direct, comparative studies will be required to determine whether gilteritinib or quizartinib is superior in specific genetic subpopulations.
As previously stated, TP53 and RAS pathway mutations are a significant major resistance factor to VEN-based regimens. Preliminary findings of the phase 1/2 APTIVATE (NCT03850574) trial that enrolled R/R FLT3-wild-type, TP53- and RAS-mutant AML patients show that tuspetinib, a new multikinase inhibitor, is well tolerated and increases survival of this cohort [70, 71]. We are also waiting for results from tuspetinib + AZA + VEN arm.
IDH1- and IHD2-inhibitors
Either HMA + VEN or AZA + an IDH-inhibitor (Fig. 2) are currently the preferred options for elderly N/D patients unfit for IC. Whether AZA + VEN vs AZA + an IDH-inhibitor vs the HMA + IDH-inhibitor + VEN triplet is best for these patients remains an important clinical question. Furthermore, there is no evidence to suggest that frontline AZA + VEN followed by IDH-inhibitor + AZA in case of relapses is superior to the reverse order for IDH1/2 mutated AML. The DATA-I trial (NCT05401097) is currently recruiting subjects to answer this question [72].
A IDH role in DNA methylation. While wild-type (WT) IDH leads to normal DNA methylation and physiological production of alfa-ketoglutarate, mutant forms of IDH can lead to aberrant secretion of 2-hydroxy-glutarate (2-HG) and modified DNA methylation leading to a block in cell differentiation. The use of IDH-inhibitors in IDH mutant cells restores the normal DNA methylation pattern B The possible use of a triplet therapy including venetoclax, hypomethylating agents and IDH-inhibitors. The outcome would be a hypomethylated DNA, normal methylation due to IDH-inhibition and triggered apoptosis.
The phase 1/2 NCT03471260 trial was the first study to demonstrate increased efficacy of ivosidenib + VEN + -AZA combination. The triplet led to higher median PFS (not reached, 95% CI 3.9-not reached vs 11 months, 95% CI 22.9–not reached, P = 0.058), but without significant difference in MRD-negative CR rates (75 vs 50%), IDH1 clearance (86 vs 43%) and OS (42.1 months vs not reached, P = 0.13). FLT3-TKD, N/KRAS, NF1, PTPN11, JAK/STAT, KIT, and CSF3R mutations have been associated with worse outcomes. MRD-negativity rate increased with increasing number of cycles, median IDH1-clearance occurred in cycle 4 and MRD-positivity in previous cycles has not been associated with worse outcomes, suggesting that MRD testing in later cycles might confer higher prognostic value [73]. The latest trial update indicates that the triplet may result in better long-term outcomes, although more follow-up and comparative, prospective trials are required [74].
Enasidenib, an IDH2-inhibitor, proves as well superior when combined with VEN. NCT04092179 investigated safety and efficacy in R/R patients and achieved an ORR of 70% with a median OS of 9.4 months (95% CI, 8.2 - not reached). R172 mutated patients achieved better response rates compared to R140 (ORR of 83 vs 55%) [75].
All-oral combinations may include IDH-inhibitors as well. The NCT04774393 combines ASTX727 + VEN with ivosidenib or enasidenib for IDH-mutated AML patients unfit for IC. Preliminary data showed high efficacy with 91% of N/D and 67% of R/R patients achieving MRD-negativity by flow cytometry [76]. A pooled analysis that compared ivosidenib + VEN + AZA vs the all-oral triplets showed no significant difference in terms of outcomes and safety [77].
The phase 1/2 NCT06445959 and NCT04603001 studies are investigating novel-generation IDH-inhibitors for R/R IDH-mutated AML not eligible for IC, such as olutasidenib or the dual IDH1- and IDH2-inhibitor crelosidenib. We are awaiting the first preliminary results.
Menin-inhibitors
Menin inhibitors are a novel class of targeted therapies designed to disrupt the interaction between menin and NPM1, MLL1, NUP98, or KMT2A mutated proteins. The landscape of AML treatment is changing, with menin-inhibitors providing a less toxic option to classic therapies. These drugs may lead to responses in heavily pretreated populations, too. Currently, only preliminary data are available and only in R/R AML. The KOMET-007 trial (NCT05735184) investigated ziftomenib + AZA + VEN and achieved CR in 9 of 11 NPM1-mutated and 5 of 13 KMTA2-rearranged AML patients. Prior VEN exposure did not seem to influence efficacy [78]. Additionally, the ZiVA trial (NCT06397027) is evaluating ziftomenib + AZA + VEN in children and young adults (ages 2–30) with lower VEN exposure (14 days/cycle) [79]. SAVE (NCT05360160) investigated the all-oral revumenib + ASTX727 + VEN triplet achieving an ORR of 88% (23/26) with 17/23 patients reaching MRD-negativity by flow cytometry [80]. Revumenib + AZA + VEN is also tested in young (<30 years) R/R patients in the NCT06177067 trial. New generation menin-inhibitors, such as bleximenib or emilumenib, showed high synergism with VEN in vivo [81]. Clinical studies (NCT05453903, NCT04752163) for patients with R/R AML have been started to investigate these novel agents with an AZA + VEN backbone.
Currently, the KOMET-007 trial is the only one comparing the “7 + 3” IC regimen with ziftomenib to the ziftomenib + AZA + VEN triplet in N/D patients. Preclinical evidence suggests that menin inhibitors combined with FLT3-inhibitors + VEN might be one of the most effective and active therapies for N/D AML patients [82]. Prospective trials to investigate this triplet are warranted.
MRD-based addition of menin-inhibitors may also be a feasible approach in the future. The phase 2 NCT06284486 trial evaluates consolidation with the revumenib + VEN doublet in case of MRD-positivity (based on flow cytometry) following IC or low-intensity induction therapies [83].
P53 reactivators and MDM2-inhibitors
MDM2 is an E3 ubiquitin ligase that binds to p53, causing its ubiquitination and proteasomal destruction. In AML, MDM2 overexpression or hyperactivity results in a functional p53 deficit, allowing leukemic cells to survive and proliferate [84]. MDM2 inhibitors (idasanutlin, navtemadlin and siremadlin) are molecules designed to disrupt the interaction between p53 and MDM2 and restore its tumor suppressive effects. Combination of p53 reactivators with VEN is a promising approach to increase the survival of patients with TP53 wild-type, R/R AML.
The phase 2 NCT02670044 investigated the combination of idasanutlin with VEN in R/R subjects ineligible for IC. The CR rate of the doublet, according to preliminary data, was 26% (13/50) with a median OS of 5.1 months (95% CI, 3.4–7.3). Patients with IDH1/2 or RUNX1 mutations achieved higher CR rates even in the presence of TP53 mutations. N/KRAS, FLT3, CBL, NF1, and PTPN1 mutations were associated with worse outcomes. Another limitation of the idasanutlin + VEN doublet may be the selective pressure for preexisting TP53-mutated clones [85]. Siremadlin, a novel-generation MDM2 inhibitor, has also been tested for R/R AML in combination with VEN in the NCT03940352 trial. Preliminary data reported four patients of ten achieving CR without any significant toxicities, cytopenias and febrile neutropenia being the most common adverse events [86]. The NCT05155709 trial is also testing siremadlin in a triplet combination with AZA and VEN for N/D patients unfit for IC without any TP53 mutations [87].
Eprenetapopt (APR-246) is a first-in-class small-molecule p53 reactivator that induces apoptosis also in TP53-mutated leukemic cells and could be the first FDA-approved targeted therapy for TP53-mutated AML. The NCT04214860 trial reported impressive outcomes with an ORR of 64% (25/39) and a median OS of 7.3 months (95% CI 5.6–9.8) without any treatment-related mortality. An indirect comparison of N/D patients revealed that the triplet achieved higher rates and longer-lasting responses than AZA + VEN alone [88].
Cyclin-dependent kinase (CDK)-inhibitors
Dysregulated CDK activity is involved in leukemogenesis, particularly through its role in driving proliferation, evading apoptosis, and maintaining aberrant transcriptional programs. CDKs have emerged as attractive targets in AML to disrupt these pathological processes, restore cell cycle control and induce apoptosis. Upregulation of MCL-1, an antiapoptotic protein, plays a crucial role in the development of VEN resistance in AML. Alvocidib, a CDK9-inhibitor, downregulates MCL-1, and previous studies indicated that it synergizes with VEN [89]. The phase 1 NCT03441555 study aimed to evaluate the safety and preliminary efficacy of the alvocidib-VEN combination in adults with R/R AML. The study noted that responses (only 20% of patients) were observed primarily in patients with no prior exposure to VEN and overall, concluded that the combination did not yield sufficient efficacy to warrant further investigation [90]. Voruciclib is a new CDK9-inhibitor that led to responses in 10 of 32 (31%) patients in the NCT03547115 trial. However, only two of them obtained CR [91]. Triplet combinations on an AZA + VEN backbone are being investigated for many novel generation, potent CDK9-inhibitors that may improve outcomes. Preliminary data from the Chinese phase 2 trial NCT06532058 showed that 6 of the 18 evaluable patients achieved CR, with an ORR of 72.2% and 3 obtained MRD-negativity. The combination appears to induce responses in TP53-mutated AML as well [92]. Further evaluation is warranted.
Selective inhibitors of nuclear export (SINEs)
Selinexor is a first-in-class selective inhibitor of nuclear export that targets exportin-1 (XPO1), a nuclear transport protein required for cellular homeostasis. By inhibiting XPO1, selinexor promotes the nuclear retention and activation of tumor suppressor proteins such as p53 while decreasing levels of antiapoptotic proteins, including MCL-1 [93]. This dual mechanism of action makes selinexor a promising agent to combine with VEN. Preliminary data of the phase 1 NCT05736965 trial reported high efficacy of the selinexor + AZA + VEN triplet in N/D patients ineligible for IC, with an ORR of 90% (18/20) and 16/20 achieving CR, with 3 patients being MRD-negative [94]. Several trials are currently enrolling R/R patients as well to assess the efficacy of selinexor combined with AZA + VEN. Eltanexor, a second-generation compound, is also tested with AZA + VEN for R/R AML in the phase 1 NCT06399640. We are waiting for the first preliminary results.
Other targeted therapies
Recent advancements in the understanding of leukemogenesis have driven the development of novel targeted therapies, including tyrosine kinase inhibitors and agents targeting critical signaling pathways and epigenetic regulators. Table 3 summarizes the studies with targeted therapies that we identified. RAS pathway mutations are frequently associated with VEN resistance.
According to preclinical data, mitogen-activated protein kinase (MAPK)-inhibitors, such as trametinib or cobimetinib synergize with VEN and overcome VEN resistance [95]. NCT04487106 is a phase 1 trial that investigated the efficacy of trametinib in combination with VEN in R/R AML patients. Unfortunately, there was no improvement in outcomes, and the trial was terminated [96]. Similar results have been observed in NCT02670044 with cobimetinib [97]. Bromodomain and extraterminal (BET) family proteins are epigenetic regulators that play a role in leukemogenesis by upregulating antiapoptotic molecules. BET-inhibitors, such as mivebresib, have been tested in combination with VEN to more efficiently decrease the levels of BCL-xL, BCL-2 and upregulate proapoptotic/sensitizer peptides BIM and PUMA, which eventually would lead to higher apoptotic rates of leukemic cells. However, the NCT02391480 trial failed to demonstrate the efficacy of the combination [98].
Pevonedistat, a first-in-class inhibitor of the NEDD8-activating enzyme, disrupts the neddylation process, leading to decreased survival of leukemic cells through mechanisms such as reduced NF-κB activity and increased apoptosis. This approach aims to enhance the therapeutic potential of AZA + VEN. Results of the NCT03862157 trial showed a CR rate of 66% in the secondary AML cohort, with a median OS of 8.1 months. Therefore, the triplet combination demonstrates promising efficacy in secondary AML and warrants further evaluation [99]. In R/R AML, high efficacy, although in a small number of subjects, was demonstrated in five of seven patients, achieving CR with four subjects reaching MRD-negativity by flow cytometry. These findings suggest that adding pevonedistat to AZA + VEN might improve outcomes in heavily pretreated patients, and prospective trials assessing efficacy in specific genetic subgroups are needed [100].
Inflammation, oncogenesis, and cancer cell survival are all driven, among others, by interleukin-1 receptor-associated kinase 4 (IRAK4). An isoform of IRAK4 is overexpressed due to mutations in the splicing factors SF3B1 and U2AF1, which have been associated with worse outcomes in AML. The NCT04278768 trial investigated the efficacy of a novel IRAK4-inhibitor, CA-4948, in combination with AZA + VEN for R/R AML patients. Preliminary data show that two of five patients with spliceosome mutations achieved long-standing CR and were able to proceed to transplant [101].
Uproleselan (GMI-1271) is a new, first-in-class inhibitor of E-selectin, a cell adhesion protein that is essential in the tumor microenvironment of AML. E-selectin is overexpressed in the bone marrow vascular niche, where it promotes leukemic cell adhesion, chemoresistance, and immune evasion by promoting interaction between leukemic and bone marrow stromal cells. These interactions increase MRD rates, which contribute to relapse. The phase 1 NCT04964505 trial enrolled N/D AML patients unfit for IC and assessed the efficacy of uproleselan + VEN. CR has been achieved in 11 of 16 patients, nine of these also reached MRD-negativity. Therefore, the combination showed high antileukemic activity and needs further evaluation [102].
Ruxolitinib targets JAK2, a key kinase involved in the JAK-STAT signaling pathway, which is often dysregulated in hematologic malignancies, including AML. The combination of ruxolitinib and VEN is being explored in the NCT03874052 clinical trial. Preliminary data indicate that the combination is safe; nonetheless, only modest efficacy (10% CR rate and a median OS of only 3.7 months, 95% CI 2.3–6.5) has been described, with CD56-negativity being a predictive biomarker of response [103]. STAT-inhibitors, such as danvasertib or OPB-111077, are also investigated in the phase 1 NCT05986240 and NCT03063944 trials. Only one of seven patients achieved CR, and three died due to progressive disease [104].
Immune checkpoint-inhibitors
Pembrolizumab, a programmed death-1 (PD1) inhibitor, combined with AZA or DEC + VEN, has been tested in both N/D and R/R patients in the NCT04284787 and NCT03969446. Preliminary data for N/D participants showed no increase in efficacy; nevertheless, worse outcomes were observed with the triplet, leading to early discontinuation of the trial [105]. Inhibition of PD1-ligand by avelumab in NCT03390296 did not bring any survival benefit either [106].
Magrolimab is a monoclonal antibody targeting CD47, designed to enhance macrophage-mediated phagocytosis of cancer cells by blocking the “don’t eat me” signal. NCT04435691 investigated the efficacy of magrolimab combined with the AZA + VEN backbone. High CR rates have been achieved in TP53-mutated N/D patients (N = 17) but with modest long-term outcomes (median OS = 7.6 months). CD47 upregulation and the inflammatory tumor microenvironment seem to be the main resistance mechanisms [107]. A phase 3 trial (ENHANCE-3, NCT05079230) to evaluate the combination has been designed but it was terminated due to increased risk of death in the magrolimab-arm.
Sabalotimab is an anti-TIM-3 monoclonal antibody, a new generation checkpoint-inhibitor, designed to enhance immune responses by targeting T-cell exhaustion. The STIMULUS-01 trial (NCT04150029) is currently enrolling N/D AML patients not suitable for IC to investigate sabalotimab in combination with AZA + VEN. According to initial preliminary data, the triplet is safe [108]. Additionally, we are awaiting the first efficacy reports.
Novel strategies to activate the immune system include downregulation of galectin-9 by the fully human monoclonal antibody, LYT-200. Preliminary data of the NCT05829226 trial showed that combination of LYT-200 with AZA + VEN in R/R AML led to 1 CR and 6 stable diseases of the 8 evaluable patients [109]. Further efficacy analysis is warranted.
In AML, a highly immunosuppressive tumor microenvironment, characterized by dysfunctional T-cells, regulatory T-cell expansion, and myeloid-derived suppressor cell infiltration, provides a rationale for targeting immune checkpoints. Preclinical data suggested that their combination with VEN may enhance antileukemic activity [110]. Currently available trials, however, fail to demonstrate any clinical benefit of adding VEN to checkpoint inhibitors. Another drawback worth considering is the substantial toxicity associated with checkpoint inhibitors, which mostly target barrier organs such as the gastrointestinal tract (colitis), skin (rash, dermatitis), lungs (pneumonitis), and liver (immune hepatitis). Up to 30% of patients may also present with endocrine system involvement, including thyroiditis, hypophysitis, and type 1 diabetes [111]. Combining checkpoint-inhibitors with other chemotherapy agents increased the risk of hypotension, myocarditis and arrhythmias [112]. The most concerning aspect, however, when combined with VEN, is the increased risk of developing hematologic toxicities, the most prevalent of which following checkpoint-inhibitor therapies are severe anemia (0.1–17%) and thrombocytopenia (1.2–2.5%) [113].
Monoclonal antibodies and antibody-drug conjugates
Efficacy of the gemtuzumab ozogamicin, an anti-CD33 monoclonal antibody, and VEN doublet is currently tested in a phase 1 study NCT04070768. Efficacy of gemtuzumab + AZA + VEN proved a promising combination in NCT03390296, with 11 of 21 (52%) of treated R/R AML patients achieving CR or morphologic leukemia-free states with a median OS of 7.6 months. Poor-risk cytogenetics did not predict statistically different survival. Prior exposure to VEN, however, led to statistically significant decrease in responses (P = 0.02) [106].
Cusatuzumab is an anti-CD70 monoclonal antibody, another potential immunotherapy candidate, that targets leukemia stem cells and enhances anti-tumor immunity in AML. The phase 1 CULMINATE trial (NCT04023526) demonstrated modest efficacy of cusatuzumab in combination with AZA alone [114]. Thus, two other trials (NCT04150887 - ELEVATE and NCT06384261 - OV-AML-1231) have been initiated to test the antibody with AZA + VEN in N/D AML patients ineligible for IC. Preliminary data demonstrate an improved response rate (CR in 77.3%, N = 44) and high MRD-negativity rate (47% of the responders) [115].
Antibody-drug conjugates in AML, such as lintuzumab-225Ac, pivekimab, and tagraxofusp, represent novel immune therapies designed to deliver cytotoxic agents directly to leukemia cells, improving specificity and reducing off-target effects. Preclinical models demonstrated VEN resistance may be overcome by downregulation of MCL-1 by lintuzumab-225Ac, a monoclonal antibody targeting CD33 coupled with actinium-225 (225Ac), a potent cytotoxic, alpha-emitting radionuclide [116]. The phase 1/2 NCT03867682 trial investigated this combination and preliminary data showed high activity, two of three patients achieving morphologic leukemia-free status [117].
The first-in-class tagraxofusp and pivekimab sunirine are antibody-drug conjugates that target CD123. While tagraxofusp is linked to a diphtheria toxin, pivekimab sunirine contains a DNA alkylator payload (indolinobenzodiazepine pseudodimer). Although further research and head-to-head comparisons are necessary, preliminary results suggest that tagraxofusp combined with AZA + VEN improves outcomes in high-risk AML patients, particularly those with TP53 mutations. The phase 1 NCT03113643 trial reported achievement of CR in 69% of the overall population and 54% of TP53-mutated AML subjects (N = 26). Median OS for TP53 wild-type patients was not yet reached, with a median PFS of 13.3 months (95% CI, 8.6-not reached), while in the case of TP53-mutated AML, median PFS was 5.1 months (95% CI, 1.8-not reached) with median OS of 9.5 months (95% CI, 1.8-not reached), respectively. MRD-negativity by flow cytometry has been reached in four of seven TP53-mutated patients (57%) in CR. There have been no significant safety issues reported, with the exception of reversibly elevated liver enzymes. CD123 expression levels were not correlated with the duration or depth of response [118]. Subjects are now being recruited for a phase 2 trial (NCT06456463) with a modified treatment schedule (tagraxofusp is administered on days 4–6 instead of 1–3, and VEN is administered for 28 days instead of 21 days). Pivekimab sunirine on an AZA + VEN backbone achieved similar response rates for N/D patients unfit for IC. Of the 26 of 50 (52%) of patients in CR, 19 (73%) achieved MRD-negativity. No PFS or OS data have been published yet [119].
Other targetable antigens in AML include CD47, death receptor 5 and nectin-1. These surface molecules are involved in leukemic cell survival, immune evasion, and proliferation. Table 4 summarizes clinical trials investigating monoclonal antibodies against the aforementioned antigens. No data on safety or efficacy has been published yet.
Immune cell-engagers and adoptive cellular therapies
Adoptive cellular therapies, including chimeric antigen receptor (CAR) T-cells and T-cell receptor (TCR)-engineered cells, have shown limited efficacy in AML due to challenges such as heterogeneous antigen expression, an immunosuppressive microenvironment, and relapse mechanisms. NK-cell-based adoptive cellular therapies, however, demonstrated favorable safety profiles and encouraging efficacy in AML, with response rates ranging from 25 to 88% even in patients unfit for allotransplantation [120]. Therefore, allogeneic NK-cells administered with AZA + VEN may represent an alternative approach to improve survival in elderly patients. ADVENT-AML (NCT05834244) is the first trial to investigate cellular therapy in a frontline setting in AML. Additionally, this research would provide information on the efficacy of lymphodepletion with AZA + VEN or the synergy shown between adoptive therapies and VEN. NCT06152809 is investigating the impact of allogeneic NK-cells in combination with AZA + VEN post-induction, during consolidation. No results have been published yet.
SAR443579, a natural killer (NK)-cell engager with three binding domains, one to CD123 on the surface of leukemic cells and two others to NKp46 and CD16a on NK-cells, is another novel strategy to overcome the immunosuppressive niche by activating immune cells via direct blast-immune cell crosslinking. NCT06508489 is currently recruiting patients to test this agent in combination with AZA + VEN for N/D AML.
The role of biomarkers to predict responses to venetoclax-based combinations
The European LeukemiaNet (ELN) 2022 risk classification proved to be efficient in stratifying patients given IC [7]. Döhner et al., however, reported that this classification system is not accurately prognostic of patients receiving HMA + VEN [121]. To improve the prognostic scores of patients receiving HMA + VEN, the Beat-AML score added a mutation score based on the presence of IDH2, KRAS, MLL2, and TP53 mutations, redefining AML patients who were earlier categorized as adverse risk based on ELN 2022 in an intermediate (−1 or 0 point) and an adverse risk group (1+ points). KRAS, MLL2, and TP53 mutations were unfavorable (+1 point each); however, IDH2 was regarded as an independent positive prognostic factor (−1 point) [122]. Another ELN 2024, four-gene (FLT3-ITD, KRAS, NRAS, and TP53) molecular prognostic risk signature (mPRS) has also been developed and classifies patients into three prognostic groups [123]. A retrospective study at MD Anderson Cancer Center in Houston has confirmed that the median PFS and OS are considerably different using the ELN 2024 mPRS: 19 and 30 months for the higher benefit, 8 and 12 months for the intermediate benefit (FLT3-ITD, NRAS or KRAS-mutated), and 4 and 5 months for the lower benefit (TP53-mutated) groups (P < 0.001) [124]. While the Beat-AML and the ELN 2024 mPRS are predicting survival, the Mayo score has been developed as a predictor of treatment response and categorized patients in four molecular signatures based on a six-gene panel (NPM1, IDH2, DDX41, TP53, RUNX1, and FLT3-ITD). In the Mayo risk model, survival was superior in patients with IDH2 R172K vs. R140Q mutations (not reached vs. 19.2 months, P = 0.02) [125]. KMT2A rearrangements, presence of a PTPN11 mutation, monocytic or secondary AML and male gender have also been identified as risk factors for a worse prognosis [126]. Novel prognostic scores that incorporate MRD-status, although not currently used in the clinic, are also needed in the future.
Conclusion
Venetoclax has emerged as a game changer in the treatment landscape of AML. VEN combined with targeted therapies or epigenetic regulators like HMA, FLT3-, IDH-, or menin-inhibitors, as well as immunotherapies (monoclonal antibodies, antibody-drug conjugates, or adoptive cellular therapies) not only improves efficacy but holds the potential to overcome resistance mechanisms that frequently limit monotherapy. Therefore, basic and translational research to identify novel resistance mechanisms is vital.
VEN-based combination therapies are tailored to balance efficacy with safety. Which combination is best and whether the addition of multiple agents is beneficial, and if so, the optimal dose, treatment duration and disease subtypes remain to be determined. Development of a mutation-guided therapeutic pathway is one of the most significant challenges in modern clinical practice due to the many important questions that remain to be answered. For instance, for patients with FLT3-mutations alone, the experience with triple therapy emerging from clinical trials as well as from real-world experience is that the use of gilteritinib + HMA + VEN is very toxic and the high degree of cytopenias is that makes the combination difficult to use. Perhaps using the lower duration of VEN (7 or 14 days) may be sufficient to make it more tolerable. An alternative approach is to use the drugs sequentially. For instance, given the fact that FLT3-mutant clones at diagnosis represent a relatively small fraction of the malignant cells, we might initiate HMA + VEN for 14 or 21 days and start gilteritinib only after day 21 or 28. Subsequent cycles can use either 7 or 14 days of VEN. If a patient has both FLT3-ITD and IDH mutations, what shall one use for induction/consolidation is another unanswered problem. Generally, IDH-mutations confer resistance to FLT3-inhibitors and thus, a combo of FLT3-ITD + IDH mutations may be less likely to respond to FLT3-inhibitors, particularly at diagnosis. Thus, one could use FLT3-inhibitors only in consolidation/maintenance/relapse. On the other hand, IDH-mutant AML cells are more sensitive to VEN (IDH1 mutants are less so than IDH2). Thus, for IDH1-mutant AML, one may consider using IDH1-inhibitors rather than HMA + VEN, while for the IDH2-mutant disease, perhaps the use of HMA + VEN may be more likely to offer clinical benefits. The bottom line is that there is no standardized approach for these clinical situations. Perhaps novel clinical trial designs and artificial intelligence algorithms should predict the best sequence of therapies based on the type of mutation, the variant allele frequency of mutations, the molecular interactions between the signaling programs activated downstream of the mutations, but also based on drug and reimbursement availability in various health care systems, as well as clinical toxicities.
The increased turnaround time for genomic and other molecular tests creates a further clinical dilemma about whether empiric/early treatment should be undertaken. A comprehensive registry-based analysis of 854 AML patients treated with VEN-based combinations, however, indicates that there is no significant difference in response rates or mortality when treatment is initiated within the first 10 days of diagnosis or later. Therefore, delaying treatment for optimal and personalized treatment selection may become a viable approach in the clinic since better clinical monitoring and risk analysis may be performed [127].
While the clinical outcomes are encouraging, challenges remain, particularly regarding the identification of predictive biomarkers to guide patient selection. The use of novel prognostic scores, such as the Beat-AML, the Mayo or the ELN 2024 classifications, are an important step towards precision therapy. Future research should prioritize well-designed clinical trials, real-world studies, and mechanistic insights to refine these combinations and expand their applicability. Further trials are needed to assess optimal duration of treatment, but we are optimistic that future clinical trials will confirm that decreasing VEN exposure to 14 or 21 days, or even to 7 days, and HMA exposure to 5 or 3 days provides a significant clinical benefit [121].
Furthermore, there is now an unmet need for the development of novel-generation BCL-2 inhibitors that can target both MCL-1 and BCL-xL without causing any major cardiac or hematologic toxicity. WH25244, for instance is a novel BCL-2 and BCL-xL inhibitor that spares platelets and acts on mutant, hyperphosphorylated BCL-2 molecules that showed high cytotoxicity in vitro. Further preclinical and clinical studies are needed to confirm its efficacy [128].
Overall, the combinations presented in this manuscript represent a paradigm shift in AML management, paving the path for longer-lasting remissions and better patient outcomes. Continued innovations and collaboration will be essential to harness the potential of declaring this currently incurable disease a curable one.
References
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–2.
Puthier D, Pellat-Deceunynck C, Barillé S, Robillard N, Rapp MJ, Juge-Morineau N, et al. Differential expression of Bcl-2 in human plasma cell disorders according to proliferation status and malignancy. Leukemia. 1999;13:289–94.
Huang JZ, Sanger WG, Greiner TC, Staudt LM, Weisenburger DD, Pickering DL, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99:2285–90.
Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–55.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Shimony S, Stahl M, Stone RM. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am J. Hematol. 2023;98:502–26.
Lachowiez CA, DiNardo CD, Loghavi S. Molecularly targeted therapy in acute myeloid leukemia: current treatment landscape and mechanisms of response and resistance. Cancers. 2023;15:1617.
Bhansali RS, Pratz KW, Lai C. Recent advances in targeted therapies in acute myeloid leukemia. J Hematol Oncol. 2023;16:29.
Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6:1106–17.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Venditti A, Hou JZ, Fenaux P, Jonas BA, Vrhovac R, Montesinos P, et al. Outcomes in chemotherapy-ineligible elderly patients with newly diagnosed acute myeloid leukemia treated with venetoclax plus azacitidine: a pooled analysis. Blood. 2023;142:2886.
Pratz KW, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Döhner H, et al. Long-term follow-up of VIALE-A: venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am J. Hematol. 2024;99:615–24.
Chen S, Xie J, Yang X, Shen H, Cen J, Yao L, et al. Venetoclax plus decitabine for young adults with newly diagnosed ELN adverse-risk acute myeloid leukemia: interim analysis of a prospective, multicenter, single-arm, phase 2 trial. Blood. 2021;138:35.
Venugopal S, Maiti A, DiNardo CD, Loghavi S, Daver NG, Kadia TM, et al. Decitabine and venetoclax for IDH1/2 - mutated acute myeloid leukemia. Am J Hematol. 2021. https://onlinelibrary.wiley.com/doi/10.1002/ajh.26122.
Johnson IM, Ilyas R, McCullough K, Al-Kali A, Alkhateeb HB, Begna K, et al. Molecular predictors of response and survival in patients with relapsed/refractory acute myeloid leukemia following venetoclax plus hypomethylating agent therapy. Blood. 2022;140:3233–4.
Gangat N, Ilyas R, Johnson IM, McCullough K, Al-Kali A, Alkhateeb HB, et al. Outcome of patients with acute myeloid leukemia following failure of frontline venetoclax plus hypomethylating agent therapy. Haematologica. 2023;108:3170–4.
Stahl M, Menghrajani K, Derkach A, Chan A, Xiao W, Glass J, et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021;5:1552–64.
Jin D, He J, Chen H, Wu W, Han X, Le J, et al. Impact of monocytic differentiation on acute myeloid leukemia patients treated with venetoclax and hypomethylating agents. Cancer Med. 2024;13:e7378.
DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7:e724–36.
Watts JM, Sohalski C, Zoso A, Dell-Martin J, Belachew A, Abbott D, et al. Venetoclax plus azacitidine for newly diagnosed younger acute myeloid leukemia patients independent of fitness for intensive chemotherapy. Blood. 2024;144:969.
Kretschmer L, Ruhnke L, Schliemann C, Fransecky L, Steffen B, Kaufmann M, et al. Trial in progress: a randomized-controlled phase 2 study evaluating venetoclax plus azacitidine versus intensive chemotherapy in adult patients with newly diagnosed, NPM1 -mutated AML - the SAL/AMLCG Vincent trial. Blood. 2024;144:1527.1.
Bewersdorf JP, Shimony S, Shallis RM, Liu Y, Berton G, Schaefer EJ, et al. Intensive induction chemotherapy vs hypomethylating agents in combination with venetoclax in NPM1 -mutant AML. Blood Adv. 2024;8:4845–55.
Bewersdorf JP, Shimony S, Shallis RM, Liu Y, Berton G, Schaefer EJ, et al. Combination therapy with hypomethylating agents and venetoclax versus intensive induction chemotherapy in IDH1 - or IDH2 -mutant newly diagnosed acute myeloid leukemia—A multicenter cohort study. Am J. Hematol. 2024;99:1640–3.
Wan CL, Liu YQ, Liu FT, Huang YH, Cao HY, Huang SM, et al. Venetoclax with hypomethylating agents versus intensive chemotherapy in newly diagnosed acute myeloid leukemia with myelodysplasia related changes: a propensity score matched analysis based on international consensus classification. Blood. 2024;144:1527–1527.
Aguirre LE, Bewersdorf JP, Liu Y, Shallis RM, Boussi L, Zucenka A, et al. Comparative analysis of outcomes with HMA plus venetoclax vs intensive chemotherapy in AML patients harboring very-high risk cytogenetics. Blood. 2024;144:4267.
Boussi L, Bewersdorf JP, Liu Y, Shallis RM, Aguirre LE, Zucenka A, et al. Outcomes with HMA plus venetoclax vs intensive chemotherapy in AML patients with chromosome 5 and 7 abnormalities. Blood. 2024;144:4281.
Salhotra A, Aribi A, Ngo D, Zhang J, Sandhu K, Al-Malki M, et al. Outcome of secondary acute myeloid leukemia treated with hypomethylating agent plus venetoclax (HMA-Ven) or liposomal daunorubicin-cytarabine (CPX -351). Am J Hematol. 2021. https://onlinelibrary.wiley.com/doi/10.1002/ajh.26157.
Little RF, Othus M, Assouline S, Ansher S, Atallah EL, Lindsley RC, et al. Umbrella trial in myeloid malignancies: the myelomatch national clinical trials network precision medicine initiative. Blood. 2022;140:9057–60.
Lu J, Xue S, Wang Y, Dai HP, He X, Hu X, et al. Comparing the efficacy and safety of venetoclax combined with decitabine versus conventional chemotherapy as induction therapy for young adults with newly diagnosed acute myeloid leukemia - interim analysis of a multicenter, randomized, phase 2b trial. Blood. 2023;142:970.
Karrar O, Abdelmagid M, Rana M, Iftikhar M, McCullough K, Al-Kali A, et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: comparative analysis of response, toxicity, and survival. Am J Hematol. 2024. https://onlinelibrary.wiley.com/doi/10.1002/ajh.27180.
Boisclair S, Naing PT, Zhou E, Thakur R, Jou E, Goldberg B, et al. Outcomes and hospital resource utilization associated with decreased ven exposure in acute myeloid leukemia patients: a real-world retrospective review. Blood. 2023;142:4268.
Boisclair S, Zhou E, Naing P, Thakur R, Jou E, Goldberg B, et al. Less is more: an analysis of venetoclax and hypomethylating agent post-induction treatment modifications in AML. Leuk Res. 2024;143:107545.
Cui J, Chen X, Li C, Yan Q, Yuan G. Reduced duration and dosage of venetoclax is efficient in newly diagnosed patients with acute myeloid leukemia. Hematology. 2024;29:2293512.
Ginosyan AA, Ashouri K, Humayun L, Ford L, Baya M, Hong HJ, et al. Optimizing venetoclax duration in combination with hypomethylating agents for newly diagnosed AML: impact on treatment response and survival outcomes. Blood. 2024;144:2892.
Willekens C, Bazinet A, Chraibi S, Bataller A, Decroocq J, Arani N, et al. Reduced venetoclax exposure to 7 days vs standard exposure with hypomethylating agents in newly diagnosed AML patients. Blood Cancer J. 2025;15:68.
Gangat N, Tefferi A. Venetoclax schedule in AML: 7 vs 14 vs 21 vs 28 days. Blood Cancer J. 2025;15:56.
Suo X, Ma X, Zheng F, Wang D, Bai G, Zhao L, et al. Venetoclax combined with three-day multi-frequency decitabine (DEC3-VEN) in elder or intensive chemotherapy ineligible patients with newly diagnosed acute myeloid leukemia. Blood Cancer J. 2024;14:204.
Bazinet A, Kantarjian H, Bataller A, Pemmaraju N, Borthakur G, Chien K, et al. Reduced dose azacitidine plus venetoclax as maintenance therapy in acute myeloid leukaemia following intensive or low-intensity induction: a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2024;11:e287–98.
Goldfinger M, Mantzaris I, Shastri A, Saunthararajah Y, Gritsman K, Sica RA, et al. A weekly low-dose regimen of decitabine and venetoclax is efficacious and less myelotoxic in a racially diverse cohort. Blood. 2024;144:2360–3.
Pratz KW, Jonas BA, Pullarkat V, Recher C, Schuh AC, Thirman MJ, et al. Measurable residual disease response and prognosis in treatment-naïve acute myeloid leukemia with venetoclax and azacitidine. J Clin Oncol. 2022;40:855–65.
Gutman JA, Winters A, Kent A, Amaya M, McMahon C, Smith C, et al. Higher-dose venetoclax with measurable residual disease-guided azacitidine discontinuation in newly diagnosed acute myeloid leukemia. Haematologica. 2023;108:2616–25.
Carre M, Meunier M, Gross Z, Aspas Requena G, Tauveron-Jalenques U, Tavernier E, et al. Azacytidine and venetoclax as a salvage therapy in measurable residual disease (MRD) relapse post intensive chemotherapy or allogenic stem cell transplantation in acute myeloid leukemia patients: a multicentric study from French Auraml Group. Blood. 2024;144:4335–4335.
Jamy O, Lin K, Worth S, Bachiashvili K, Rangaraju S, Vachhani P, et al. Hypomethylating agent/venetoclax versus intensive chemotherapy in adults with relapsed or refractory acute myeloid leukaemia. Br J Haematol. 2022. https://onlinelibrary.wiley.com/doi/10.1111/bjh.18229.
Manda S, Anz BM, Benton C, Broun ER, Yimer HA, Renshaw JS, et al. A phase 3b study of venetoclax and azacitidine or decitabine in an outpatient setting in patients with acute myeloid leukemia. Hematol Oncol. 2024;42:e3274.
Amaya ML, McMahon CM, Schwartz M, Gutman JA, Sohalski C, Tobin J, et al. Results from a phase (Ph) 1 clinical study of the all-oral regimen of CC-486 and venetoclax for relapsed and refractory acute myeloid leukemia. Blood. 2023;142:2914.
Fleming S, Roboz GJ, Fathi AT, Zhang TY, Wei A, Carraway HE, et al. Phase 1b OMNIVERSE trial: safety and tolerability of oral azacitidine (Oral-AZA) in combination with venetoclax (VEN) for treatment of acute myeloid leukemia. J Clin. Oncol. 2023;41:e19011.
Mannis GN, Griffiths EA, Savona MR, Odenike O, Roboz GJ, O’Connell CL, et al. A phase 1 study evaluating ASTX727 (decitabine and cedazuridine) and venetoclax combination therapy in newly diagnosed AML patients unfit for intensive induction chemotherapy. Blood. 2021;138:1245.
Bazinet A, Garcia-Manero G, Short N, Alvarado Y, Bataller A, Abuasab T, et al. Oral decitabine and cedazuridine plus venetoclax for older or unfit patients with acute myeloid leukaemia: a phase 2 study. Lancet Haematol. 2024;11:e276–86.
Bazinet A, Garcia-Manero G, Short NJ, Alvarado Valero Y, Abuasab T, Islam MR, et al. A phase 2 study of the fully oral combination of ASTX727 (decitabine/cedazuridine) plus venetoclax for older and/or unfit patients with acute myeloid leukemia. Blood. 2023;142:833.
Geissler K, Koristek Z, Bernal Del Castillo T, Novak J, Rodriguez Macias G, Metzelder S, et al. Oral decitabine/cedazuridine vs intravenous decitabine for acute myeloid leukemia: final results of a randomized, crossover, registration-enabling, pharmacokinetics study. Blood. 2023;142:1538.
Maiti A, DiNardo CD, Ohanian M, Daver N, Borthakur G, Chien KS, et al. Phase II trial of 10-day ASTX727 (decitabine/cedazuridine) with venetoclax for relapsed or refractory acute myeloid leukemia. Blood. 2023;142:4289.
Bazinet A, Kantarjian HM, Ravandi F, Short NJ, Daver N, Ohanian M, et al. Decitabine/cedazuridine (ASTX727) combined with a molecularly-targeted agent (venetoclax, gilteritinib, ivosidenib, or enasidenib) as personalized maintenance therapy in acute myeloid leukemia: first results from a phase 1b study. Blood. 2023;142:2909.
Russo D, Polverelli N, Bernardi S, Santarone S, Farina M, Borlenghi E, et al. Venetoclax plus decitabine as a bridge to allogeneic haematopoietic stem-cell transplantation in older patients with acute myeloid leukaemia (VEN-DEC GITMO): final report of a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2024;11:e830–8.
Sartor C, Candoni A, Piciocchi A, Marsili G, Fazi P, Papayannidis C, et al. Venetoclax and azacitidine for relapse prevention in NPM1 -mutated acute myeloid leukemia in molecular failure: results from the ongoing Gimema AML2521 phase 2 trial. Blood. 2024;144:2893.
Tavernier E, Cornillon J, Gross Z, Aspas Requena G, Tauveron-Jalenques U, Carré M, et al. Azacitidine and venetoclax as a salvage therapy for relapse after allogeneic transplantation for acute myeloid leukemia: a multicentric study from French Auraml Group. Blood. 2024;144:1518.
Li Z, Wang J, Ge SS, Qiu QC, Du JH, Shan SS, et al. Combination of venetoclax and midostaurin efficiently suppressed relapsed t(8;21)acute myeloid leukemia with mutant KIT after failure of venetoclax plus azacitidine treatment. Front Oncol. 2022;12:841276.
Wang ES, Montesinos P, Minden MD, Lee JH, Heuser M, Naoe T, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3 mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140:1845–57.
Daver N, Perl AE, Maly J, Levis M, Ritchie E, Litzow M, et al. Venetoclax plus gilteritinib for FLT3 -mutated relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2022;40:4048–59.
Short NJ, Daver N, Dinardo CD, Kadia T, Nasr LF, Macaron W, et al. Azacitidine, venetoclax, and gilteritinib in newly diagnosed and relapsed or refractory FLT3 -mutated AML. J Clin Oncol. 2024;42:1499–508.
Short NJ, Loghavi S, Yilmaz M, Karrar O, Jabbour JE, DiNardo CD, et al. Long-term survival outcomes and cytogenetic/molecular patterns of relapse in adults with FLT3 -mutated AML receiving frontline triplet therapy with a hypomethylating agent, venetoclax and FLT3 inhibitor. Blood. 2024;144:220.
Liu Q (Selina), Welkie RL, Huang Y, Swords RT, Lin TL, Koenig KL, et al. Beat AML S8 group 2: gilteritinib (GILT) in combination with decitabine (DEC) and venetoclax (VEN) in untreated FLT3 mutated acute myeloid leukemia (AML) patients age ≥60 with high and low variant allele frequency (VAF). Blood. 2023;142:5933.
Briski R, Short NJ, Daver N, Kadia TM, DiNardo CD, Yilmaz M, et al. A phase I/II study of combination of ASTX727, gilteritinib and venetoclax in patients with relapsed/refractory FLT3 mutated acute myeloid leukemia (AML). Blood. 2023;142:2910.
Altman JK, Sun Z, Perl AE, Little R, Gore SD, Michaelis LC, et al. A randomized phase II study of venetoclax and HMA-based therapies for the treatment of older and unfit adults with newly diagnosed FLT3-mutated acute myeloid leukemia (AML): a myelomatch treatment trial: ECOG-ACRIN MM20A-EA02. Blood. 2024;144:2907.1.
Bergua-Burgues JM, Rodríguez-Veiga R, Cano I, Vall-llovera F, García-Guiñon A, Gómez-Estruch J, et al. P512: preliminary results of Ven-a-Qui study: a phase 1-2 trial to assess the safety and efficacy of the ombination of azacitidine or low-dose cytarabine with venetoclax and quizartinib in newly diagnosed. HemaSphere. 2022;6:411–2.
Yilmaz M, Muftuoglu M, Kantarjian HM, Dinardo CD, Kadia TM, Konopleva M, et al. Quizartinib (QUIZ) with decitabine (DAC) and venetoclax (VEN) is active in patients (pts) with FLT3-ITD mutated acute myeloid leukemia (AML): a phase I/II clinical trial. J Clin Oncol. 2022;40:7036.
Yilmaz M, Muftuoglu M, Short NJ, Loghavi S, Kadia TM, DiNardo CD, et al. Phase I/II study of quizartinib, venetoclax, and decitabine triple combination in FLT3-ITD mutated AML. Blood. 2024;144:4263.
Daver N, Lee KH, Choi Y, Montesinos P, Jonas BA, Arellano ML, et al. Phase 1 safety and efficacy of tuspetinib plus venetoclax combination therapy in study participants with relapsed or refractory acute myeloid leukemia (AML) support exploration of triplet combination therapy of tuspetinib plus venetoclax and azacitidine for newly diagnosed AML. Blood. 2024;144:4255.
Daver N, Lee KH, Choi Y, Jonas BA, Arellano M, Koller PB, et al. Tuspetinib myeloid kinase inhibitor safety and efficacy as monotherapy and combined with venetoclax in phase 1/2 trial of patients with relapsed or refractory (R/R) acute myeloid leukemia (AML). Blood. 2023;142:162.
Ozga MP, Dvorak-Kornaus KM, Zhao Q, Buss J, Laganson A, Hamp E, et al. I-DATA study: randomized, sequential, open-label study to evaluate the efficacy of IDH targeted/non- targeted versus non-targeted/IDH-targeted approaches in the treatment of newly diagnosed IDH mutated adult AML patients not candidates for intensive induction therapy. Blood. 2023;142:1534.
Lachowiez CA, Loghavi S, Zeng Z, Tanaka T, Kim YJ, Uryu H, et al. A phase Ib/II study of ivosidenib with venetoclax ± azacitidine in IDH1-mutated myeloid malignancies. Blood Cancer Discov. 2023;4:276–93.
Marvin-Peek J, Garcia JS, Borthakur G, Garcia-Manero G, Short NJ, Kadia TM, et al. A phase Ib/II study of ivosidenib with venetoclax ± azacitidine in IDH1-mutated hematologic malignancies: a 2024 update. Blood. 2024;144:219.
Richard-Carpentier G, Gupta G, Cameron C, Cathelin S, Bankar A, Davidson MB, et al. Final results of the phase Ib/II study evaluating enasidenib in combination with venetoclax in patients with IDH2-mutated relapsed/refractory myeloid malignancies. Blood. 2023;142:159.
Atluri H, Mullin J, Takahashi K, Loghavi S, Maiti A, Sasaki K, et al. Phase Ib/2 study of oral decitabine/cedazuridine (ASTX727) and venetoclax in combination with the targeted mutant IDH1 inhibitor ivosidenib or the targeted mutant IDH2 inhibitor enasidenib: 2023 update. Blood. 2023;142:968.
Marvin-Peek J, Atluri H, Short NJ, Maiti A, Takahashi K, Loghavi S, et al. Clinical outcomes using frontline “Triplet” regimens for newly diagnosed IDH-mutated acute myeloid leukemia (AML): a pooled analysis of two phase Ib/2 clinical trials. Blood. 2024;144:2883.
Fathi AT, Issa GC, Wang ES, Erba H, Altman JK, Balasubramanian SK, et al. Ziftomenib combined with venetoclax/azacitidine in relapsed/refractory NPM1 -m or KMT2A -r acute myeloid leukemia: interim phase 1a results from KOMET-007. Blood. 2024;144:2880.
McCall D, Cuglievan B, Gibson A, Garcia MB, Roth ME, Nunez C. A Phase I study investigating the combination of the ziftomenib, venetoclax and azacitidine (ZiVA) in pediatric relapsed and refractory acute leukemias. Blood. 2024;144:1504.4.
Issa GC, Cuglievan B, Daver N, DiNardo CD, Farhat A, Short NJ, et al. Phase I/II study of the all-oral combination of revumenib (SNDX-5613) with decitabine/cedazuridine (ASTX727) and venetoclax (SAVE) in R/R AML. Blood. 2024;144:216.
Kwon MC, Verhulst T, Goffin D, Marien A, Verbist B, Guttke C, et al. Preclinical efficacy of the menin-KMT2A inhibitor JNJ-75276617 in combination with venetoclax and azacitidine in AML. Blood. 2023;142:4167.
Carter BZ, Mak PY, Tao W, Ostermann LB, Mak DH, Ke B, et al. Inhibition of menin, BCL-2, and FLT3 combined with a hypomethylating agent cures NPM1/FLT3-ITD/-TKD mutant acute myeloid leukemia in a patient-derived xenograft model. Haematologica. 2023. https://haematologica.org/article/view/haematol.2022.281927.
Issa GC, Ambinder AJ, Xiao W, Manalis S, Shalek AK, Gondek LP, et al. A multi-site break through cancer trial: phase II study investigating dual inhibition of BCL2 and menin in AML MRD using the combination of venetoclax and revumenib (Trial In progress). Blood. 2024;144:4265.2.
Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–9.
Daver NG, Dail M, Garcia JS, Jonas BA, Yee KWL, Kelly KR, et al. Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial. Blood. 2023;141:1265–76.
Wei AH, Breccia M, Ooi M, Cedena Romero MT, Ciceri F, Erba HP, et al. Preliminary results from a phase Ib study exploring MDM2 inhibitor siremadlin (HDM201) in combination with B-cell lymphoma-2 (BCL-2) inhibitor venetoclax in patients with acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome (HR-MDS). Blood. 2021;138:1283.
Daver N, Wei AH, Stein E, DeAngelo DJ, Pathak D, Xu Y, et al. Siremadlin in combination with venetoclax (VEN) plus azacitidine (AZA) in adult patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy: a phase Ib/II trial. Blood. 2022;140:11625–7.
Garcia-Manero G, Goldberg AD, Winer ES, Altman JK, Fathi AT, Odenike O, et al. Eprenetapopt combined with venetoclax and azacitidine in TP53-mutated acute myeloid leukaemia: a phase 1, dose-finding and expansion study. Lancet Haematol. 2023;10:e272–83.
Bogenberger J, Whatcott C, Hansen N, Delman D, Shi CX, Kim W, et al. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget. 2017;8:107206–22.
Jonas BA, Hou J, Roboz GJ, Alvares CL, Jeyakumar D, Edwards JR, et al. A phase 1b study of venetoclax and alvocidib in patients with relapsed/refractory acute myeloid leukemia. Hematol Oncol. 2023;41:743–52.
Alvarado-Valero Y, Cook RJ, Dinner SN, Keng M, Begna K, Abedin S, et al. A phase 1 study of the oral CDK9 inhibitor voruciclib in combination with venetoclax in relapsed/refractory (R/R) acute myeloid leukemia (AML). Blood. 2024;144:2878.
Wu M, Zhang Y, Wu W, Ge Z, Liu L, Ji C, et al. High remission rates in relapsed/refractory acute myeloid leukemia with QHRD107 (CDK9 Inhibitor), venetoclax, and azacitidine combination therapy (107VA regimen): preliminary results of a phase 2a study. Blood. 2024;144:2882.
Luedtke DA, Su Y, Liu S, Edwards H, Wang Y, Lin H, et al. Inhibition of XPO 1 enhances cell death induced by ABT -199 in acute myeloid leukaemia via Mcl-1. J Cell Mol Med. 2018;22:6099–111.
Yang L, Chen F, Liang H, Bai Y, Wu W, Yan X, et al. Selinexor in combination with venetoclax and azacitidine for newly diagnosed (ND) unfit acute myeloid leukemia (AML): a multicenter, open-label prospective study. Blood. 2023;142:55.
Han L, Zhang Q, Dail M, Shi C, Cavazos A, Ruvolo VR, et al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica. 2020;105:697–707.
Desikan SP, Ravandi F, Pemmaraju N, Konopleva M, Loghavi S, Jabbour EJ, et al. A phase II study of azacitidine, venetoclax, and trametinib in relapsed or refractory acute myeloid leukemia harboring RAS pathway-activating mutations. Acta Haematol. 2022;145:529–36.
Konopleva MY, Dail M, Daver NG, Garcia JS, Jonas BA, Yee KWL, et al. Venetoclax and cobimetinib in relapsed/refractory AML: a phase 1b trial. Clin Lymphoma Myeloma Leuk. 2024;24:364–74.
Borthakur G, Odenike O, Aldoss I, Rizzieri DA, Prebet T, Chen C, et al. A phase 1 study of the pan-bromodomain and extraterminal inhibitor mivebresib (ABBV-075) alone or in combination with venetoclax in patients with relapsed/refractory acute myeloid leukemia. Cancer. 2021;127:2943–53.
Short NJ, Muftuoglu M, Ong F, Nasr L, Macaron W, Montalban-Bravo G, et al. A phase 1/2 study of azacitidine, venetoclax and pevonedistat in newly diagnosed secondary AML and in MDS or CMML after failure of hypomethylating agents. J Hematol Oncol. 2023;16:73.
Murthy GSG, Saliba AN, Szabo A, Harrington A, Abedin S, Carlson K, et al. A phase I study of pevonedistat, azacitidine, and venetoclax in patients with relapsed/refractory acute myeloid leukemia. Haematologica. 2024. https://haematologica.org/article/view/haematol.2024.285014.
Garcia-Manero G, Winer ES, DeAngelo DJ, Tarantolo SR, Sallman DA, Dugan J, et al. Phase 1/2a study of the IRAK4 inhibitor CA-4948 as monotherapy or in combination with azacitidine or venetoclax in patients with relapsed/refractory (R/R) acute myeloid leukemia or lyelodysplastic syndrome. J Clin Oncol. 2022;40:7016.
Jonas BA, Karanjawala ZE, Molnar L, Dang-Chu AL, Abedi M, Esteghamat NS, et al. Updated results of a phase I study of uproleselan combined with azacitidine and venetoclax for the treatment of older or unfit patients with treatment naïve acute myeloid leukemia. Blood. 2024;144:1503.
Borate UM, Madanat YF, Tognon C, Mishra S, Kaempf A, Patel PA, et al. Results of a phase 1 trial testing the novel combination therapy of venetoclax and ruxolitinib in relapsed/refractory acute myeloid leukemia patients. Blood. 2023;142:1515.
Wilde L, Martinez-Outschoorn U, Palmisiano N, Keiffer G, Kasner M. Results of the phase 1b dose escalation study of OPB-111077, decitabine, and venetoclax for the treatment of newly diagnosed or relapsed/refractory AML. Blood. 2020;136:10.
Stempel JM, Uy GL, Dinner SN, Gojo I, Reed D, Roy R, et al. Efficacy and safety of pembrolizumab added to azacitidine plus venetoclax for patients with acute myeloid leukemia: results from an investigator-initiated, multi-center, CTEP-sponsored randomized, phase II trial (BLAST AML-2). Blood. 2024;144:736.
Short NJ, Borthakur G, Pemmaraju N, Dinardo CD, Kadia TM, Jabbour E, et al. A multi-arm phase Ib/II study designed for rapid, parallel evaluation of novel immunotherapy combinations in relapsed/refractory acute myeloid leukemia. Leuk Lymphoma. 2022;63:2161–70.
Arora S, Senapati J, Loghavi S, Reville PK, Wang B, Kadia TM, et al. Phase 1b/2 study of magrolimab (Magro), azacitidine (AZA) and venetoclax (VEN) in patients (pts) with newly diagnosed (ND) older/unfit or high risk acute myeloid leukemia (AML) and relapsed refractory (R/R) AML: final clinical data and genomic markers of resistance/relapse. Blood. 2024;144:735.
Zeidan AM, Westermann J, Kovacsovics T, Assouline S, Schuh AC, Kim HJ, et al. AML-484 first results of a phase II study (STIMULUS-AML1) investigating sabatolimab + azacitidine + venetoclax in patients with newly diagnosed acute myeloid leukemia (ND AML). Clin Lymphoma Myeloma Leuk. 2022;22:S255.
Fathi AT, Filipovic A, Maher K, Schaar DG, Niroula R, Maly JJ, et al. A phase I dose escalation and expansion trial of Lyt-200 (a first-in-class anti-galectin-9 antibody) alone and in combination with venetoclax/HMA in relapsed/refractory AML/MDS. Blood. 2024;144:1499.
Kohlhapp FJ, Haribhai D, Mathew R, Duggan R, Ellis PA, Wang R, et al. Venetoclax increases intratumoral effector T cells and antitumor efficacy in combination with immune checkpoint blockade. Cancer Discov. 2021;11:68–79.
Keam S, Turner N, Kugeratski FG, Rico R, Colunga-Minutti J, Poojary R, et al. Toxicity in the era of immune checkpoint inhibitor therapy. Front Immunol. 2024;15:1447021.
Zhang C, Wei F, Ma W, Zhang J. Immune-related cardiovascular toxicities of PD-1/PD-L1 inhibitors in solid tumors: an updated systematic review and meta-analysis. Front Immunol. 2024;15:1255825.
Kroll MH, Rojas-Hernandez C, Yee C. Hematologic complications of immune checkpoint inhibitors. Blood. 2022;139:3594–604.
Pabst T, Papayannidis C, Demirkan F, Doronin V, Fogliatto LM, Guttke C, et al. Cusatuzumab plus azacitidine in newly diagnosed acute myeloid leukaemia ineligible for intensive chemotherapy (CULMINATE): part one of a randomised, phase 2, dose optimisation study. Lancet Haematol. 2023;10:e902–12.
Roboz GJ, Pabst T, Aribi A, Brandwein JM, Döhner H, Fiedler W, et al. Safety and efficacy of cusatuzumab in combination with venetoclax and azacitidine (CVA) in patients with previously untreated acute myeloid leukemia (AML) who are not eligible for intensive chemotherapy; an open-label, multicenter, phase 1b study. Blood. 2021;138:369.
Garg R, Allen KJH, Dawicki W, Geoghegan EM, Ludwig DL, Dadachova E. 225Ac-labeled CD33-targeting antibody reverses resistance to Bcl-2 inhibitor venetoclax in acute myeloid leukemia models. Cancer Med. 2021;10:1128–40.
Sloboz GJ, Finn LE, Orozco JJ, Hegazi M, Desai A, Chen M, et al. Updated results from phase 1 study of targeted radiotherapy with lintuzumab-Ac225 in combination with venetoclax in relapsed/refractory AML. Blood. 2023;142:1539.
Lane AA, Garcia JS, Raulston EG, Garzon JL, Galinsky I, Baxter EW, et al. Phase 1b trial of tagraxofusp in combination with azacitidine with or without venetoclax in acute myeloid leukemia. Blood Adv. 2024;8:591–602.
Daver N, Montesinos P, Altman JK, Wang ES, Martinelli G, Roboz GJ, et al. Pivekimab sunirine (PVEK, IMGN632), a CD123-targeting antibody-drug conjugate, in combination with azacitidine and venetoclax in patients with newly diagnosed acute myeloid leukemia. Blood. 2023;142:2906.
Maiti A, Muftuoglu M, Ignatz-Hoover JJ, DiNardo CD, Ravandi F, Andreeff M, et al. Azacitidine, venetoclax and allogeneic NK cells in newly diagnosed acute myeloid leukemia (ADVENT-AML): an investigator-initiated multicenter phase Ib trial. Blood. 2023;142:4863.
Döhner H, Pratz KW, DiNardo CD, Jonas BA, Pullarkat VA, Thirman MJ, et al. ELN risk stratification is not predictive of outcomes for treatment-naïve patients with acute myeloid leukemia treated with venetoclax and azacitidine. Blood. 2022;140:1441–4.
Hoff FW, Blum WG, Huang Y, Welkie RL, Swords RT, Traer E, et al. Beat-AML 2024 ELN–refined risk stratification for older adults with newly diagnosed AML given lower-intensity therapy. Blood Adv. 2024;8:5297–305.
Döhner H, Pratz KW, DiNardo CD, Wei AH, Jonas BA, Pullarkat VA, et al. Genetic risk stratification and outcomes among treatment-naive patients with AML treated with venetoclax and azacitidine. Blood. 2024;144:2211–22.
Bataller A, Bazinet A, DiNardo CD, Maiti A, Borthakur G, Daver NG, et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 2024;8:927–35.
Gangat N, Elbeih A, Ghosoun N, McCullough K, Aperna F, Johnson IM, et al. Mayo genetic risk models for newly diagnosed acute myeloid leukemia treated with venetoclax + hypomethylating agent. Am J Hematol. 2025;100:260–71.
Griffioen MS, De Leeuw DC, Janssen JJWM, Smit L. Targeting acute myeloid leukemia with venetoclax; biomarkers for sensitivity and rationale for venetoclax-based combination therapies. Cancers. 2022;14:3456.
Baden D, Zukunft S, Hernandez G, Wolgast N, Steinhauser S, Pohlmann A, et al. Time from diagnosis to treatment has no impact on survival in newly diagnosed acute myeloid leukemia treated with venetoclax-based regimens. Haematologica. 2024. https://haematologica.org/article/view/haematol.2024.285225.
Diaz Rohena DY, Mitchell AD, Wang J, Chamberlain S, Liu C, Sanchez JR, et al. Protein degrader WH25244 eliminates venetoclax resistance factors: mutant or hyperphosphorylated BCL2, and BCL-XL. Blood. 2024;144:2786.
Zhou Z, Tan Y, Zhao X, Suo X, Bai G, Bai Y, et al. Venetoclax combined with three-day multi-frequency decitabine (DEC3-VEN) in the treatment of adult patients with de novo acute myeloid leukemia. Blood. 2024;144:4279.
Aiba M, Shigematsu A, Suzuki T, Miyagishima T. Shorter duration of venetoclax administration to 14 days has same efficacy and better safety profile in treatment of acute myeloid leukemia. Ann Hematol. 2023;102:541–6.
Kanaya M, Onozawa M, Matsukawa T, Miyashita N, Fujii F, Yoshida S, et al. Evaluation of venetoclax duration in venetoclax-azacitidine regimen for untreated acute myeloid leukemia: real world experience from Hokkaido leukemia net. Blood. 2024;144:6063.
Mirgh S, Sharma A, Shaikh MRMA, Kadian K, Agrawal N, Khushoo V, et al. Hypomethylating agents+venetoclax induction therapy in acute myeloid leukemia unfit for intensive chemotherapy - novel avenues for lesser venetoclax duration and patients with baseline infections from a developing country. Am J Blood Res. 2021;11:290–302.
Acknowledgements
DK is funded by a national research grant of the Romanian Government—Bursa Henri Coanda, contract 8/10.02.2024. CT is funded by an international grant of the European Hematology Association (EHA-SWG Immunotherapy Project 2024—CAR NK-cells for tumor associated macrophage immunomodulation—a new era of immunotherapy), by an international grant of the European Commission—Horizon Europe Framework Network, HORIZON-TMA-MSCA-DN, Proposal Number 101227725—"Advancing in the CHallenge of Improving Lymphoma and LEukemia Survival— ACHILLES”, as well as by a bilateral collaboration grant between Romania and Moldova (PN-IV-P8-8.3-ROMD-2023-0036). HE is funded by a national grant of the Romanian Research Ministry—PNRR 2024-2026 (PNRR/2022/C9/MCID/18, Contract No. 760278/26.03.2024).
Author information
Authors and Affiliations
Contributions
DK, AT, ASV, DVT, RI, ABT, DC, AB, SI, CJ, MN, and RT wrote the manuscript. DG, MZ, and HE synthesized the data and provided the revision. HE, GG, CC, and CT supervised the work and provided the financial support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kegyes, D., Tat, A., Vizitiu, A.S. et al. Comprehensive view on chemotherapy-free management of acute myeloid leukemia by using venetoclax in combination with targeted and/or immune therapies. Cell Death Discov. 11, 379 (2025). https://doi.org/10.1038/s41420-025-02678-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-025-02678-4