Abstract
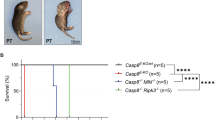
The hypersecretion of cytokines triggers life-threatening systemic inflammatory response syndrome (SIRS), leading to multiple organ dysfunction syndrome (MODS) and mortality. Although both coagulopathy and necroptosis have been identified as important factors in the pathogenesis of SIRS, the specific cell types that undergo necroptosis and the interrelationships between coagulopathy and necroptosis remain unclear. In this study, we utilized visualization analysis via intravital microscopy to demonstrate that both anticoagulant heparin and nonanticoagulant heparin (NAH) pretreatment protect mice against TNF-α-induced mortality in SIRS. Moreover, the deletion of Mlkl or Ripk3 resulted in decreased coagulation and reduced mortality in TNF-α-induced SIRS. These findings suggest that necroptosis plays a key role upstream of coagulation in SIRS-related mortality. Furthermore, using a genetic lineage tracing mouse model (Tie2-Cre;Rosa26-tdT), we tracked endothelial cells (ECs) and verified that EC necroptosis is responsible for the vascular damage observed in TNF-α-treated mice. Importantly, Mlkl deletion in vascular ECs in mice had a similar protective effect against lethal SIRS by blocking EC necroptosis to protect the integrity of the endothelium. Collectively, our findings demonstrated that RIPK3–MLKL-dependent necroptosis disrupted vascular integrity, resulting in coagulopathy and multiorgan failure, eventually leading to mortality in SIRS patients. These results highlight the importance of targeting vascular EC necroptosis for the development of effective treatments for SIRS patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8.
Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth. 2010;2:161–75.
Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401–10.
Salgado A, et al. Inflammatory mediators and their influence on hemostasis. Hemostasis. 1994;24:132–8.
Dunzendorfer S, et al. Pentoxifylline differentially regulates migration and respiratory burst activity of the neutrophil. Ann N Y Acad Sci. 1997;832:330–40.
Delvaeye T, et al. Noninvasive whole-body imaging of phosphatidylethanolamine as a cell death marker using 99mTc-duramycin during TNF-induced SIRS. J Nucl Med. 2018;59:1140–5.
Gando S, et al. Activation of the extrinsic coagulation pathway in patients with severe sepsis and septic shock. Crit Care Med. 1998;26:2005–9.
Gando S, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17:R297.
van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54:2450–64.
Ruan Q, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8.
Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4.
Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195–203.
Li C, et al. The programmed cell death of macrophages, endothelial cells, and tubular epithelial cells in sepsis-AKI. Front Med. 2021;8:796724.
Rangel-Frausto MS, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–23.
Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44.
Ogura H, et al. Sirs-associated coagulopathy and organ dysfunction in critically Ill patients with thrombocytopenia. Shock. 2007;28:411–7.
Esmon CT. Inflammation and thrombosis. J Thromb Hemost. 2003;1:1343–8.
Gando S, et al. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb Hemost. 1996;75:224–8.
Iba T, et al. Coagulopathy in COVID‐19. J Thromb Hemost. 2020;18:2103–9.
Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021;47:100761.
Guo F, et al. Clinical applications of machine learning in the survival prediction and classification of sepsis: coagulation and heparin usage matter. J Transl Med. 2022;20:265.
Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–55.
Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90.
Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5.
Chen ZJ. Ubiquitin signaling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–65.
Vandenabeele P, et al. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–14.
Bertrand MJM, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700.
Kovalenko A, et al. The tumor suppressor CYLD negatively regulates NF-kappaB signaling by deubiquitination. Nature. 2003;424:801–5.
Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703.
Dickens LS, et al. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol Cell. 2012;47:291–305.
Murphy JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–53.
Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27.
Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2013;16:55–65.
Duprez L, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–18.
Polykratis A, et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in Vivo. J Immunol. 2014;193:1539–43.
Newton K, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–60.
Berger SB, et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–80.
Newton K, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–76.
Zelic M, et al. RIP kinase 1–dependent endothelial necroptosis underlies systemic inflammatory response syndrome. J Clin Investig. 2018;128:2064–75.
Wu C, et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. 2019;50:1401–11.e4.
Grover SP, Mackman N. Tissue Factor. Arterioscler Thromb Vasc Biol. 2018;38:709–25.
Fajgenbaum DC, Longo DL, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73.
Karki R, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–68.e17.
Wada T, et al. Disseminated intravascular coagulation with increased fibrinolysis during the early phase of isolated traumatic brain injury. Crit Care. 2017;21:219.
Kenig M, et al. Identification of the heparin-binding domain of TNF-alpha and its use for efficient TNF-alpha purification by heparin-Sepharose affinity chromatography. J Chromatogr B Anal Technol Biomed Life Sci. 2008;867:119–25.
Kuschert GS, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–68.
Beurskens DMH, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Hemost. 2020;120:1371–83.
Tang Y, et al. Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity. 2021;54:454–67.e6.
Zhang H, et al. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin Transl Med. 2023;13:e1170.
Chakraborty RK, Burns B. Systemic inflammatory response syndrome. In: StatPearls©. Treasure Island, FL: StatPearls Publishing LLC; 2022.
Gómez-Jiménez J, et al. L-arginine: nitric oxide pathway in endotoxemia and human septic shock. Crit Care Med. 1995;23:253–8.
Zhang H, et al. Crucial roles of the RIP homotypic interaction motifs of RIPK3 in RIPK1-dependent cell death and lymphoproliferative disease. Cell Rep. 2020;31:107650.
Zhao Q, et al. RIPK3 mediates necroptosis during embryonic development and postnatal inflammation in Fadd-deficient mice. Cell Rep. 2017;19:798–808.
Tennant M, McGeachie JK. Blood vessel structure and function: a brief update on recent advances. Aust N Z J Surg. 1990;60:747–53.
Zhang S, et al. Seamless genetic recording of transiently activated mesenchymal gene expression in endothelial cells during cardiac fibrosis. Circulation. 2021;144:2004–20.
Zeng W, et al. Effect of drotrecogin alfa (activated) on human endothelial cell permeability and Rho kinase signaling. Crit Care Med. 2004;32:S302–S308.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20.
Van Hauwermeiren F, et al. Safe TNF-based antitumor therapy following p55TNFR reduction in intestinal epithelium. J Clin Investig. 2013;123:2590–603.
Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518.
Sturtzel C. Endothelial Cells. Adv Exp Med Biol. 2017;1003:71–91.
Chen A-Q, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain–barrier disruption after ischemic stroke. Cell Death Dis. 2019;10:487.
Bolik J, et al. Inhibition of ADAM17 impairs endothelial cell necroptosis and blocks metastasis. J Exp Med. 2022;219:e20201039.
Yang L, et al. TAK1 regulates endothelial cell necroptosis and tumor metastasis. Cell Death Differ. 2019;26:1987–97.
Wang L, et al. Multi-arm PEG/peptidomimetic conjugate inhibitors of DR6/APP interaction block hematogenous tumor cell extravasation. Adv Sci. 2021;8:e2003558.
Fritsch M, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–7.
Zou C, et al. Reduction of mNAT1/hNAT2 contributes to cerebral endothelial necroptosis and Aβ accumulation in Alzheimer’s disease. Cell Rep. 2020;33:108447.
Huang W-Y, et al. TNFα-mediated necroptosis in brain endothelial cells as a potential mechanism of increased seizure susceptibility in mice following systemic inflammation. J Neuroinflammation. 2022;19:29.
Fan C, et al. Lack of FADD in Tie-2 expressing cells causes RIPK3-mediated embryonic lethality. Cell Death Dis. 2016;7:e2351.
Yang X, et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity. 2019;51:983–96.e6.
Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell. 2016;165:668–78.
Acknowledgements
We thank Dr. Xiaodong Wang (National Institute of Biological Sciences, Beijing, China) for providing Ripk3−/− mice. We also thank Zhonghui Weng (Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences) for animal studies, Lin Qiu (Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences) for flow cytometry technical support, and Yanqing Qin (Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences) for complete blood count analysis.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2022YFA0807300), the National Natural Science Foundation of China (32270803, 32300630, 82272181, T2293734), the Shanghai Excellent Academic/Technical Leader Program (22XD1404500), the Shanghai Science and Technology Commission (23141902800), and the China Postdoctoral Science Foundation (2020M671261). We also acknowledge support from the Shanghai Municipal Science and Technology Major Project and the GuangCi Professorship Program of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Author information
Authors and Affiliations
Contributions
Xiaoxia Wu and Haibing Zhang designed the study and analyzed the data; Xiaoxia Wu performed all the experiments with assistance from XMZ, FL, YW, YJO, HWZ, XML, XHW, LXW, ML, JLL, MYX, HL, YYW, YYX, and HWZ; YW and JW provided technical support for SD-IVM. YZ and YCT assisted with mouse genotyping and analysis. Yan Luo, LMS, HL, and Yu Li provided resources and intellectual input. Xiaoxia Wu and Haibing Zhang assembled the figure panels and wrote the paper. Haibing Zhang supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Zhao, X., Li, F. et al. MLKL-mediated endothelial necroptosis drives vascular damage and mortality in systemic inflammatory response syndrome. Cell Mol Immunol 21, 1309–1321 (2024). https://doi.org/10.1038/s41423-024-01217-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-024-01217-y
Keywords
This article is cited by
-
Cumulative intra-abdominal pressure exposure and dynamic trajectories in ICU-admitted patients reveal prognostic determinants of severe acute pancreatitis
World Journal of Emergency Surgery (2025)
-
Research advances on the role of programmed endothelial cell death in sepsis
Cell Death Discovery (2025)
-
Necroptotic cell death consequences and disease relevance
Nature Immunology (2025)
-
Opposing regulation of the K63-linked polyubiquitination of RIPK3 by SMURF1 and USP5 in necroptosis
Nature Communications (2025)