Abstract
Nanocelluloses, which are prepared from natural cellulose sources in a top-down manner through physical and/or chemical treatments, are broadening the scope of applications of sustainable biopolymers. These naturally derived nanocelluloses exhibit one-dimensional nanomorphologies, such as nanofibers and nanorods, which originate from the intrinsic nanostructures formed by cellulose molecules in plants and other cellulose-producing organisms. Recent studies have developed artificial nanocelluloses that are constructed in vitro at the molecular level via the self-assembly of low-molecular-weight (LMW) cellulose. These artificial nanocelluloses feature unique nanostructures, including rectangular nanosheets, square nanosheets, distorted nanosheets, and helical nanorods. Nevertheless, most artificial nanocelluloses reported to date are particulates. We have developed two types of nanostructured macroscopic materials through the self-assembly of LMW cellulose: nanoribbon network hydrogels and nanospiked microfibrous materials. These novel nanostructured cellulose materials have shown great promise for distinctive applications of cellulose. This Focus Review summarizes our work along with related studies on nanostructured macroscopic materials constructed via the self-assembly of LMW cellulose.
Similar content being viewed by others
Introduction
Cellulose is the most abundant organic material on Earth and is a representative sustainable biopolymer [1, 2]. This polysaccharide has been utilized as a versatile material since ancient times and remains widely used today in the form of paper, cloth, and other products. Conventional cellulosic materials are typically composed of microfibrous structures. In the 21st century, nanocelluloses have been developed as novel cellulosic materials [3,4,5]. Cellulose nanofibers (CNFs) and cellulose nanocrystals (CNCs) are the two most representative types, obtained from natural cellulose sources through physical and/or chemical treatments. These nanocelluloses possess one-dimensional nanomorphologies—nanofibers in the case of CNFs and nanorods in the case of CNCs—which originate from the intrinsic nanostructures formed by cellulose molecules in plants and other cellulose-producing organisms. In other words, naturally derived nanocelluloses have a limited variety of nanomorphologies.
Artificial nanocelluloses represent an emerging class of nanocelluloses that have been developed over the past decade [6,7,8,9]. These novel nanocelluloses are composed of low-molecular-weight (LMW) cellulose with a degree of polymerization (DP) of approximately 10 and are produced via self-assembly in vitro. While high-molecular-weight cellulose has traditionally been used to construct assembled materials (so-called regenerated cellulose) [6, 10, 11], LMW cellulose exhibits minimal entanglement of molecular chains during self-assembly, facilitating the formation of well-ordered, highly crystalline nanostructures. The artificial nanocelluloses reported to date include rectangular nanosheets [12, 13], square nanosheets [14, 15], distorted nanosheets [16], and helical nanorods [16]. Although the self-assembly of LMW cellulose into nanostructures has been investigated for more than 50 years, it is only in the past decade that LMW cellulose assemblies have gained significant attention as artificial nanocelluloses in the field of materials science [9].
The self-assembly of LMW cellulose for artificial nanocellulose production is typically conducted either through the precipitation of LMW cellulose from supersaturated solutions or by chemical synthesis under precipitation polymerization conditions [9]. Notably, these self-assembly processes generally yield particulate artificial nanocelluloses that sediment during formation. This behavior appears to result from the strong intermolecular interactions among cellulose chains, which promote aggregation and limit further growth into well-developed assembled structures.
We have developed two types of nanostructured macroscopic materials through self-assembly of LMW cellulose (Fig. 1). One is a nanoribbon network hydrogel, and the other is a nanospiked microfibrous material. Nanoribbon network hydrogels are produced by suppressing the aggregation and sedimentation of LMW cellulose assemblies during self-assembly, thereby enabling their further growth into a network structure that results in macroscopic gel formation [17,18,19,20,21,22,23,24,25]. Nanospiked microfibrous materials are obtained via self-assembly of LMW cellulose in conventional microfibrous materials (e.g., paper or gauze), where the LMW cellulose assembles at the surface of the microfibers and adopts a nanospike morphology [26, 27]. In this Focus Review, I summarize our research and related studies on these novel nanostructured macroscopic materials composed of LMW cellulose.
Nanostructured macroscopic materials fabricated through the self-assembly of LMW cellulose. a Hydrogels composed of nanoribbon-shaped lamellar crystals of LMW cellulose. Adapted with permission from ref. [17]. Copyright 2017 American Chemical Society. b Gauze with nanospike structures of LMW cellulose. Adapted from ref. [27]. Copyright 2025 The Authors
LMW cellulose as a self-assembling building block
One of the most attractive characteristics of cellulose as a self-assembling building block is its insolubility in water and most common organic solvents [28,29,30]. This property arises from the molecular structure of cellulose (Fig. 2) [28, 31,32,33,34]. The polysaccharide chain tends to adopt a ladder-like conformation because of the fully equatorial arrangement of β-linked glucopyranose residues and the formation of intrachain hydrogen bonds between the O3–H and O5 atoms. This conformation leads to the accumulation of C–H groups on the molecular plane and O–H groups at the periphery, endowing cellulose with amphiphilic character. This unique amphiphilicity enables cellulose chains to interact strongly with each other via hydrophobic effects in water and hydrogen bonding in low-polarity solvents. Moreover, the rigidity of the molecular chain reduces the entropy gain upon dissolution, and the crystalline solid state of cellulose is highly stable. These features collectively contribute to the low solubility of cellulose.
Although the solubility of cellulose tends to increase as its molecular weight decreases, even oligomeric forms remain insoluble in water. Generally, cello-oligosaccharides with a DP of six or less are water soluble, whereas those with a DP of seven or more are substantially insoluble in water at room temperature [35,36,37]. Consequently, LMW cellulose can form irreversible assemblies in aqueous environments. Owing to this irreversibility, LMW cellulose assemblies can be classified as nanocelluloses, despite the significantly lower molecular weights of their components compared to those of naturally derived nanocelluloses [9]. Typically, LMW cellulose used for artificial nanocelluloses has a DP of approximately 10, which ensures sufficient insolubility.
Despite their high insolubility, the surfaces of artificial nanocelluloses expose the terminal saccharide residues of LMW cellulose chains, which present numerous hydroxy groups and exhibit significant hydrophilicity. The hydrophilic surfaces of artificial nanocelluloses confer antibiofouling properties, which are characterized by suppressed protein adsorption and cell adhesion [38,39,40,41]. This is in stark contrast to most water-insoluble polymers and oligomers, which typically expose hydrophobic surfaces. It is noted that antibiofouling properties have been reported exclusively for nanosheet-shaped lamellar crystals (see below) but not for other types of artificial nanocelluloses. The unique combination of water insolubility and surface hydrophilicity—seemingly contradictory properties—makes artificial nanocelluloses particularly attractive for applications in fields such as medicine and environmental science.
There are two major self-assembly systems for the preparation of artificial nanocelluloses: precipitation of LMW cellulose from supersaturated solutions and chemical synthesis under precipitation polymerization conditions [9]. The former is carried out, for example, by adding a coagulant to LMW cellulose solutions. The latter employs enzymatic oligomerization reactions to synthesize LMW cellulose in an aqueous solution, during which the growing molecular chains, once they become water insoluble, undergo self-assembly.
The most common form of artificial nanocellulose is rectangular, nanosheet-shaped lamellar crystals, in which the LMW cellulose chains are aligned in an antiparallel manner and oriented perpendicular to the plane of the nanosheets [12, 13, 36, 42, 43]. The crystal allomorph is cellulose II, which is the most stable allomorph of cellulose [1, 44]. Nanosheet-shaped artificial nanocelluloses have been produced via various self-assembly processes, such as the deacetylation of LMW cellulose acetate in aqueous methylamine at 90 °C [45], the precipitation of LMW cellulose from supercritical water [15], and the cellodextrin phosphorylase-catalyzed synthesis of LMW cellulose [12, 13].
Nanoribbon network hydrogels
We have demonstrated that suppressing the aggregation and sedimentation of rectangular nanosheet-shaped lamellar crystals during their formation enables further crystal growth into nanoribbons in bulk solution, resulting in hydrogel formation (Figs. 1a and 3). As stated above, the cellodextrin phosphorylase-catalyzed synthesis of LMW cellulose typically yields nanosheet-shaped lamellar crystals as sediment [12, 13]. It appears that the nanosheets aggregate during their formation due to the strong intermolecular interactions of cellulose. In contrast, when enzymatic synthesis was carried out under macromolecular crowding conditions, created by using dextran, poly(ethylene glycol), or other water-soluble polymers at relatively high concentrations (≥10%), hydrogels composed of nanoribbon-shaped lamellar crystals of LMW cellulose formed [17, 18, 46]. Analyses suggested that the so-called crowding effects, namely, reduced diffusion rates and depletion stabilization, kinetically suppressed the aggregation of LMW cellulose assemblies, allowing the nanosheet-shaped lamellar crystals to further grow into nanoribbons, which physically contacted each other to form network structures. The nanoribbon network hydrogels appeared translucent (i.e., not opaque), suggesting a relatively homogeneous structure with minimal aggregation. Despite their low solid content (<1%), the hydrogels exhibited Young’s moduli of approximately 1 kPa.
Various polymers, including thermoresponsive gelatin, can act as crowding agents. The cellodextrin phosphorylase-catalyzed synthesis of LMW cellulose is typically carried out at 50 °C or higher, as the enzyme is derived from thermophilic bacteria [7]. At such temperatures, gelatin is in a dissolved state and functions effectively as a crowding agent [17]. The presence of dissolved gelatin during the enzymatic reaction promoted the self-assembly of LMW cellulose into nanoribbon network hydrogels. The ability of LMW cellulose to self-assemble into nanoribbon networks even in the presence of gelatin suggests its strong tendency toward self-sorting assembly. Upon subsequent cooling, the gelatin molecules underwent physical crosslinking, resulting in a double-network hydrogel composed of both nanoribbon networks of LMW cellulose and gelatin networks. The double-network hydrogel exhibited a Young’s modulus of approximately 10 kPa, which exceeded the sum of the individual component moduli.
Not only dissolved polymers but also insoluble nanomaterials can act as crowding agents. When the cellodextrin phosphorylase-catalyzed reaction was performed in aqueous dispersions of CNCs (naturally derived nanocellulose with a nanorod shape) [19] or graphene oxides [22, 23], the synthesized LMW cellulose self-assembled into nanoribbon networks. These water-dispersible nanomaterials appeared to physically suppress the sedimentation of LMW cellulose nanosheets. As a result of network formation, hybrid hydrogels composed of LMW cellulose nanoribbons and nanomaterials were produced. Notably, CNCs acted as reinforcing fillers to improve the stiffness of the hydrogels [19].
LMW cellulose nanosheets can themselves create crowded environments (i.e., self-crowding states) under appropriate conditions, enabling the formation of nanoribbon network hydrogels without the use of additional crowding agents [20]. When the temperature for the cellodextrin phosphorylase-catalyzed reaction was decreased from the conventional 60 °C to 30 °C, nanoribbon network hydrogels were obtained. Analyses suggested that the synergy between the reduced hydrophobic effect at a lower temperature and the simultaneously induced self-crowding was key to suppressing nanosheet aggregation. Notably, the hydrogels produced without additional crowding agents appeared opaque, indicating the presence of aggregated structures. Self-crowding seemed to be less effective at suppressing aggregation than macromolecular crowding. Nevertheless, the absence of additional crowding agents is advantageous in terms of easier production and higher purity of nanoribbon network hydrogels.
Other methods for producing nanoribbon hydrogels have been reported by our group and others. Remarkably, all these methods share a common concept: promoting network formation before sedimentation occurs. This concept can be implemented in two ways. One approach is to suppress sedimentation, similar to the effect achieved through crowding. Examples include the use of organic solvents [21] and ionic liquids [47] as additives that potentially increase the solvation of LMW cellulose assemblies; the accumulation of soluble oligomers that may adsorb onto LMW cellulose assemblies [48]; the use of low-gravity environments [49]; and the introduction of repulsive functional groups (e.g., oligo(ethylene glycol) and carboxylate groups) to nanosheet surfaces [50, 51]. The other approach involves the rapid precipitation of LMW cellulose, which has been achieved through enzymatic synthesis with faster kinetics [52] and pH-triggered precipitation from alkaline solutions upon acid addition [24, 39, 53].
Application of nanoribbon network hydrogels for the confinement of nanomaterials
The nanomaterials in the hybrid hydrogels were physically restricted from aggregating by confinement within the rigid network of highly crystalline LMW cellulose nanoribbons [19, 22, 23]. This is a significant outcome because nanomaterials are generally prone to aggregation, which can diminish their nanoscale-derived functionalities. Suppressing nanomaterial aggregation through confinement within LMW cellulose networks is advantageous for harnessing the functional properties of nanomaterials for a wide range of applications.
Hydrophilic nanomaterials can be dispersed in hydrophobic media through confinement within LMW cellulose nanoribbon networks. For example, when the water solvent of hybrid hydrogels containing CNCs (a type of hydrophilic nanomaterial) was exchanged with low-polarity solvents and then with hydrophobic polymer solutions, the CNCs remained well dispersed because of the confinement effect (Fig. 4a) [19]. Notably, the structural integrity of the gels was maintained without noticeable changes in shape during the solvent exchange process owing to the rigidity and insolubility of the crystalline LMW cellulose networks. Upon subsequent drying, polymer nanocomposites were obtained, in which the well-dispersed CNCs effectively served as reinforcing fillers. These results are significant given that although CNCs are promising candidates for sustainable reinforcing fillers, these hydrophilic nanomaterials are typically difficult to disperse in hydrophobic polymers.
Applications of nanoribbon network hydrogels for the confinement of nanomaterials. a Preparation of polymer nanocomposites incorporating CNCs via confinement. Adapted with permission from ref. [19]. Copyright 2018 American Chemical Society. b DNA sensing using confined reduced graphene oxides. Adapted with permission from Ref. [23]. Copyright 2020 American Chemical Society. c Preparation of three-dimensional porous structures of reduced graphene oxides via confinement. Adapted from ref. [22] – Published by the Royal Society of Chemistry
Conversely, hydrophobic nanomaterials can be maintained in a dispersed state in water through confinement. It was found that the graphene oxides in the hybrid hydrogels were chemically reduced due to side reactions under the enzymatic reaction conditions at a relatively high temperature (60 °C) [22]. This finding indicates that the reduced graphene oxides, despite their hydrophobic nature, were retained in a dispersed state in water because of the confinement effect of LMW cellulose nanoribbon networks.
The surfaces of the confined reduced graphene oxides remained accessible to biomolecules, making them suitable platforms for deoxyribonucleic acid (DNA) sensing (Fig. 4b) [23]. A disease-related DNA was used as a model target biomolecule, along with a fluorescently labeled sensor DNA possessing the complementary sequence. In the presence of the target DNA, the sensor DNA adsorbed less onto the reduced graphene oxides within the gels than in its absence, owing to the formation of double-stranded DNA, which interacts more weakly with graphene oxides than the single-stranded form. Consequently, the target DNA was detected by measuring the fluorescence intensity of the supernatant. Notably, this sensing system remained functional even in the presence of serum owing to the favorable interactions between the sensor DNA and the confined reduced graphene oxides.
Another application of confined reduced graphene oxides is their use in constructing electrode materials (Fig. 4c) [22]. When subjected to strong chemical reduction with hydrogen iodide, the confined reduced graphene oxides self-assembled into a fine porous structure. Confinement by the nanoribbon networks appeared to suppress excessive aggregation, thereby promoting the formation of relatively small pores. The resulting porous material functioned effectively as an electrode for supercapacitors.
Nanospiked microfibrous materials
The self-assembly of LMW cellulose, including that for nanoribbon hydrogel formation, has mostly been carried out in bulk solution. In contrast, a seminal study by Serizawa and colleagues demonstrated the self-assembly of LMW cellulose bearing a terminal azidoethyl group within paper, resulting in the formation of hairy nanostructures on paper microfibers via heterogeneous nucleation [54]. Although the primary aim of the study was to introduce reactive azido groups into paper for diagnostic applications, the findings also highlight the potential of paper and other microfibrous materials as substrates for developing macroscopic materials with LMW cellulose-based nanostructures.
We demonstrated the formation of nanospike structures of LMW cellulose via self-assembly on paper and cotton gauze microfibers (Figs. 1b and 5a) [26, 27]. LMW cellulose, prepared by the hydrolysis of naturally derived cellulose using phosphoric acid, was dissolved in 85% phosphoric acid [27]. A supersaturated solution of LMW cellulose was obtained by adding water as a coagulant, and this solution was immediately applied to the cotton gauze. As a result, the LMW cellulose self-assembled into nanospike structures on the gauze microfibers. The nanospikes had diameters of several tens of nanometers, but their height could be tuned by adjusting the concentration of LMW cellulose during self-assembly without significantly affecting the diameter. The LMW cellulose constituting the nanospikes exhibited the cellulose II crystal allomorph, in contrast to the cellulose I allomorph of the gauze microfibers. This difference in crystal allomorphs suggests that the LMW cellulose assembled into nanospikes via heterogeneous nucleation on the microfiber surfaces rather than through epitaxial-like crystal growth.
Nanospiked gauze. a Schematic illustration of the self-assembly of LMW cellulose into nanospike structures on gauze microfiber surfaces. b Schematic illustration of bacterial adhesion behavior on nanospiked surfaces in the absence and presence of proteins. c Removal of P. aeruginosa from deep burn wounds using the nanospiked gauze by exploiting its bacterial adhesion-promoting effects under protein-rich conditions. Adapted from ref. [27]. Copyright 2025 The Authors
The nanospikes differed from the previously reported hairy nanostructures in terms of nanomorphology [54]. Possible factors contributing to this difference include the solvent systems used (aqueous phosphoric acid for nanospikes vs. aqueous sodium hydroxide for hairy nanostructures), the terminal groups of LMW cellulose (hydroxy for nanospikes vs. azidoethyl for hairy nanostructures), and the methods of LMW cellulose preparation (acid hydrolysis, which may yield oligomers with relatively high dispersity, for nanospikes vs. enzymatic synthesis, which may yield oligomers with relatively low dispersity, for hairy nanostructures). Additionally, our study revealed that terminal-azidated LMW cellulose self-assembled from an alkaline solution into bark-like nanostructures on polyolefin, polyester, and vinylon microfibers upon neutralization with an aqueous solution of hydrogen chloride and isopropyl alcohol [55]. These findings demonstrate that a variety of nanostructures can be constructed on polymeric material surfaces by modulating the self-assembly parameters of LMW cellulose.
We demonstrated a one-pot process for the construction of nanospiked cellulose materials, in which the LMW cellulose used for self-assembly was generated from the cellulose materials themselves via partial hydrolysis [26]. When paper was swollen with 81% phosphoric acid and incubated at 45 °C for 20 h, LMW cellulose was produced owing to the acid hydrolysis of the cellulose constituting the paper. Notably, the phosphoric acid concentration of 81%, which was slightly lower than the 85% appropriate for complete dissolution of cellulose, allowed the paper to maintain its original microfibrous structures, while partial dissolution and hydrolysis occurred. The subsequent addition of water as a coagulant triggered the self-assembly of the generated LMW cellulose, resulting in the formation of a nanospike structure on the paper microfiber surface.
Application of nanospiked gauze for microorganism control
LMW cellulose nanospikes resemble the nanoscale surface architecture observed on cicada wings, which has recently attracted attention as a source of inspiration for the development of advanced materials for infection control [56, 57]. The bactericidal activity of cicada wing nanospikes was first reported in 2012, which significantly accelerated the development of functional materials for microorganism control through nanoscale surface engineering [58]. Studies have shown that nanospikes, nanopillars, and other nanostructures can either kill bacteria by mechanically disrupting the cells or minimize bacterial adhesion by reducing the contact area with bacterial cells, depending on their nanomorphology and other structural properties [56, 57, 59, 60]. The nanostructuring strategy provides an alternative or complementary approach to chemical and physical surface functionalization for microorganism control [61, 62]. However, fabricating nanospike structures on curved polymer surfaces remains a significant challenge, despite the widespread medical use of microfibrous polymer materials, such as gauze, surgical masks, and sutures. In this context, the formation of nanospike structures on curved gauze microfibers via LMW cellulose self-assembly is a remarkable advancement.
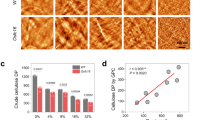
We discovered a previously unrecognized microorganism control function of nanospikes, namely, the promotion of bacterial adhesion in the presence of proteins, through the investigation of nanospiked gauze [27]. Similar to previously reported nanostructured surfaces, the nanospiked gauze exhibited low bacterial adhesion under protein-free conditions (Fig. 5b). In contrast, in the presence of proteins, the nanospike structures significantly enhanced bacterial adhesion. Analyses indicated that the adsorbed protein layers formed on the nanospike surfaces played a crucial role in this bacterial adhesion behavior.
We further harnessed this unique property of the nanospiked gauze for the adsorptive removal of pathogenic bacteria from deep burn wounds with protein-rich exudate (Fig. 5c) [27]. Specifically, a full-thickness burn injury was made in mice, inoculated with Pseudomonas aeruginosa (P. aeruginosa), and then covered with nanospiked gauze. After one day, the nanospiked gauze saturated with wound exudate was removed from the deep burn wounds. Bacterial assays revealed that the bacterial load in the wounds was significantly lower with the nanospiked gauze compared to raw gauze. These results indicate that P. aeruginosa cells preferentially adhered to the nanospiked surfaces in the presence of protein-rich wound exudate, thereby reducing the bacterial load on the wounds. In contrast to bactericidal strategies, this adhesion-based removal approach provides a promising method for infection control by capturing bacteria without killing or lysing them, thereby minimizing the release of endotoxins and other proinflammatory bacterial components.
These findings highlight the significant influence of proteins and other biomolecular species on the microorganism control functions of nanostructures. Strikingly, the effects of nanostructured surfaces on bacteria under protein-rich conditions remain largely unknown [63, 64]. Our research suggests that nanostructures may offer unexpected utility in medical applications, where materials are often in contact with body fluids containing proteins and other biomolecules.
Application of nanospiked paper for the synthesis of silver nanoparticles
Nanospiked paper was found to serve as a precursor for producing bactericidal nanocomposites containing silver nanoparticles (Fig. 6) [26]. When the nanospiked paper was subjected to autoclaving (i.e., heat treatment at 121 °C for 20 min) in silver nitrate solution, silver nanoparticles were generated on the nanospiked microfibers. The nanoparticles had diameters ranging from a few to a few tens of nanometers and were present on the nanospiked microfibers in a well-dispersed state. Analyses suggested that relatively short, hot water-soluble LMW cellulose chains dissolved from the nanospikes during the autoclaving treatment and acted as reducing agents for silver ions, leading to the synthesis of silver nanoparticles. The greater number of reducing end groups per unit mass in LMW cellulose compared to high-molecular-weight cellulose may contribute to its greater reducing ability. The resulting silver nanoparticle composites exhibited strong bactericidal activity.
Synthesis of silver nanoparticles using nanospiked paper for producing bactericidal nanocomposites. Adapted from ref. [26], Copyright 2022, with permission from Elsevier
Conclusions and outlook
This Focus Review summarized our recent progress in constructing nanostructured macroscopic materials via the self-assembly of LMW cellulose. Typically, LMW cellulose undergoes self-assembly in solution to form aggregated nanoparticles as sediment. In contrast, we have demonstrated that when aggregation and sedimentation are suppressed, LMW cellulose self-assembles into network structures composed of nanoribbon-shaped lamellar crystals. These nanoribbon network hydrogels provide a confined space that prevents the aggregation of functional nanomaterials for various applications. Furthermore, we have demonstrated that nanospike structures can be formed through heterogeneous nucleation of LMW cellulose on paper and cotton gauze microfibers. The resulting nanospiked gauze has microorganism control functions that are useful for medical applications.
Cellulose is a historically significant polymer and has been extensively studied in materials science and various other fields. Artificial nanocelluloses composed of LMW cellulose have recently emerged as a new area of cellulose research. In this context, our findings highlight a notable advantage of artificial nanocelluloses: their hierarchical structures can potentially be controlled from the nanoscale to the macroscopic scale. This level of structural tunability is uncommon for cellulosic materials, which are typically derived from natural sources while largely retaining their inherent nano- and microstructures. Nanostructured macroscopic materials based on LMW cellulose are expected to broaden the application potential of this sustainable biopolymer in materials science and industry.
References
Klemm D, Heublein B, Fink H-P, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed. 2005;44:3358–93. https://doi.org/10.1002/anie.200460587.
Li T, Chen C, Brozena AH, Zhu JY, Xu L, Driemeier C, et al. Developing fibrillated cellulose as a sustainable technological material. Nature. 2021;590:47–56. https://doi.org/10.1038/s41586-020-03167-7.
Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev. 2010;110:3479–500. https://doi.org/10.1021/cr900339w.
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, et al. Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed. 2011;50:5438–66. https://doi.org/10.1002/anie.201001273.
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40:3941–94. https://doi.org/10.1039/c0cs00108b.
Hata Y, Serizawa T. Self-assembly of cellulose for creating green materials with tailor-made nanostructures. J Mater Chem B. 2021;9:3944–66. https://doi.org/10.1039/d1tb00339a.
Nidetzky B, Zhong C. Phosphorylase-catalyzed bottom-up synthesis of short-chain soluble cello-oligosaccharides and property-tunable cellulosic materials. Biotechnol Adv. 2021;51:107633. https://doi.org/10.1016/j.biotechadv.2020.107633.
Zhong C, Nidetzky B. Bottom-up synthesized glucan materials: opportunities from applied biocatalysis. Adv Mater. 2024;36:2400436. https://doi.org/10.1002/adma.202400436.
Hata Y, Serizawa T. Nanoarchitectonics of cello-oligosaccharides: a route toward artificial nanocelluloses. Adv Colloid Interface Sci. 2025;336:103361. https://doi.org/10.1016/j.cis.2024.103361.
Kugo Y, Nomura S, Isono T, Fujiwara M, Satoh T, Tani H, et al. Crystallinity improvements in cellulose II after multiple posttreatment cycles with a dilute NaOH solution. Polym J. 2024;56:517–27. https://doi.org/10.1038/s41428-024-00890-0.
Kugo Y, Isono T, Fujiwara M, Sato T, Tani H, Erata T, et al. Optimizing crystal transitions in low-temperature, low-concentration NaOH solutions to prepare cellulose I and II composite materials. Polym J. 2024;56:939–43. https://doi.org/10.1038/s41428-024-00928-3.
Hiraishi M, Igarashi K, Kimura S, Wada M, Kitaoka M, Samejima M. Synthesis of highly ordered cellulose II in vitro using cellodextrin phosphorylase. Carbohydr Res. 2009;344:2468–73. https://doi.org/10.1016/j.carres.2009.10.002.
Serizawa T, Kato M, Okura H, Sawada T, Wada M. Hydrolytic activities of artificial nanocellulose synthesized via phosphorylase-catalyzed enzymatic reactions. Polym J. 2016;48:539–44. https://doi.org/10.1038/pj.2015.125.
Buleon A, Chanzy H. Single crystals of cellulose IVII: preparation and properties. J Polym Sci Polym Phys Ed. 1980;18:1209–17. https://doi.org/10.1002/pol.1980.180180604.
Buffiere J, Abad N, Ahvenainen P, Dou J, Cocero MJ, Sixta H. Tailoring the structure and morphology of low-molecular-weight cellulose produced during supercritical water hydrolysis. ACS Sustain Chem Eng. 2018;6:16959–67. https://doi.org/10.1021/acssuschemeng.8b04296.
Yataka Y, Sawada T, Serizawa T. Multidimensional self-assembled structures of alkylated cellulose oligomers synthesized via in vitro enzymatic reactions. Langmuir. 2016;32:10120–5. https://doi.org/10.1021/acs.langmuir.6b02679.
Hata Y, Kojima T, Koizumi T, Okura H, Sakai T, Sawada T, et al. Enzymatic synthesis of cellulose oligomer hydrogels composed of crystalline nanoribbon networks under macromolecular crowding conditions. ACS Macro Lett. 2017;6:165–70. https://doi.org/10.1021/acsmacrolett.6b00848.
Hata Y, Sawada T, Serizawa T. Effect of solution viscosity on the production of nanoribbon network hydrogels composed of enzymatically synthesized cellulose oligomers under macromolecular crowding conditions. Polym J. 2017;49:575–81. https://doi.org/10.1038/pj.2017.22.
Hata Y, Sawada T, Sakai T, Serizawa T. Enzyme-catalyzed bottom-up synthesis of mechanically and physicochemically stable cellulose hydrogels for spatial immobilization of functional colloidal particles. Biomacromolecules. 2018;19:1269–75. https://doi.org/10.1021/acs.biomac.8b00092.
Hata Y, Sawada T, Marubayashi H, Nojima S, Serizawa T. Temperature-directed assembly of crystalline cellulose oligomers into kinetically trapped structures during biocatalytic synthesis. Langmuir. 2019;35:7026–34. https://doi.org/10.1021/acs.langmuir.9b00850.
Hata Y, Fukaya Y, Sawada T, Nishiura M, Serizawa T. Biocatalytic oligomerization-induced self-assembly of crystalline cellulose oligomers into nanoribbon networks assisted by organic solvents. Beilstein J Nanotechnol. 2019;10:1778–88. https://doi.org/10.3762/bjnano.10.173.
Hata Y, Saito Y, Sawada T, Matsumoto H, Serizawa T. Assembly of reduced graphene oxides into a three-dimensional porous structure via confinement within robust cellulose oligomer networks. RSC Adv. 2019;9:38848–54. https://doi.org/10.1039/c9ra08318a.
Hata Y, Sawada T, Serizawa T. Confined reduced graphene oxides as a platform for DNA sensing in solutions crowded with biomolecules. ACS Appl Bio Mater. 2020;3:3210–6. https://doi.org/10.1021/acsabm.0c00206.
Hata Y, Kojima T, Maeda T, Sawada T, Serizawa T. pH-triggered self-assembly of cellulose oligomers with gelatin into a double-network hydrogel. Macromol Biosci. 2020;20:2000187. https://doi.org/10.1002/mabi.202000187.
Hata Y, Serizawa T. Robust gels composed of self-assembled cello-oligosaccharide networks. Bull Chem Soc Jpn. 2021;94:2279–89. https://doi.org/10.1246/bcsj.20210234.
Hata Y, Hiruma S, Sakurai Y, Sugiura K, Miyazaki H, Serizawa T, et al. Nanospiked paper: microfibrous cellulose materials nanostructured via partial hydrolysis and self-assembly. Carbohydr Polym. 2023;300:120257. https://doi.org/10.1016/j.carbpol.2022.120257.
Hata Y, Miyazaki H, Okamoto S, Serizawa T, Nakamura S. Nanospiked cellulose gauze that attracts bacteria with biomolecules for reducing bacterial load in burn wounds. Nano Lett. 2025;25:1177–84. https://doi.org/10.1021/acs.nanolett.4c05773.
Lindman B, Karlström G, Stigsson L. On the mechanism of dissolution of cellulose. J Mol Liq. 2010;156:76–81. https://doi.org/10.1016/j.molliq.2010.04.016.
Chang C, Zhang L. Cellulose-based hydrogels: present status and application prospects. Carbohydr Polym. 2011;84:40–53. https://doi.org/10.1016/j.carbpol.2010.12.023.
Wang H, Gurau G, Rogers RD. Ionic liquid processing of cellulose. Chem Soc Rev. 2012;41:1519–37. https://doi.org/10.1039/c2cs15311d.
Yamane C, Aoyagi T, Ago M, Sato K, Okajima K, Takahashi T. Two different surface properties of regenerated cellulose due to structural anisotropy. Polym J. 2006;38:819–26. https://doi.org/10.1295/polymj.PJ2005187.
Miyamoto H, Umemura M, Aoyagi T, Yamane C, Ueda K, Takahashi K. Structural reorganization of molecular sheets derived from cellulose II by molecular dynamics simulations. Carbohydr Res. 2009;344:1085–94. https://doi.org/10.1016/j.carres.2009.03.014.
Lindman B, Medronho B, Alves L, Costa C, Edlund H, Norgren M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys Chem Chem Phys. 2017;19:23704–18. https://doi.org/10.1039/c7cp02409f.
Uto T. Atomistic simulations of polysaccharide materials for insights into their crystal structure, nanostructure, and dissolution mechanism. Polym J. 2025;57:33–41. https://doi.org/10.1038/s41428-024-00966-x.
Wolfrom ML, Dacons JC. The polymer-homologous series of oligosaccharides from cellulose. J Am Chem Soc. 1952;74:5331–3. https://doi.org/10.1021/ja01141a032.
Samain E, Lancelon-Pin C, Férigo F, Moreau V, Chanzy H, Heyraud A, et al. Phosphorolytic synthesis of cellodextrins. Carbohydr Res. 1995;271:217–26. https://doi.org/10.1016/0008-6215(95)00022-L.
Zhang Y-HP, Lynd LR. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng. 2004;88:797–824. https://doi.org/10.1002/bit.20282.
Nohara T, Sawada T, Tanaka H, Serizawa T. Enzymatic synthesis and protein adsorption properties of crystalline nanoribbons composed of cellulose oligomer derivatives with primary amino groups. J Biomater Sci Polym Ed. 2017;28:925–38. https://doi.org/10.1080/09205063.2017.1322248.
Serizawa T, Maeda T, Sawada T. Neutralization-induced self-assembly of cellulose oligomers into antibiofouling crystalline nanoribbon networks in complex mixtures. ACS Macro Lett. 2020;9:301–5. https://doi.org/10.1021/acsmacrolett.9b01008.
Serizawa T, Yamaguchi S, Amitani M, Ishii S, Tsuyuki H, Tanaka Y, et al. Alkyl chain length-dependent protein nonadsorption and adsorption properties of crystalline alkyl β-celluloside assemblies. Colloids Surf B Biointerfaces. 2022;220:112898. https://doi.org/10.1016/j.colsurfb.2022.112898.
Sugiura K, Sawada T, Hata Y, Tanaka H, Serizawa T. Distinguishing anti-PEG antibodies by specificity for the PEG terminus using nanoarchitectonics-based antibiofouling cello-oligosaccharide platforms. J Mater Chem B. 2024;12:650–7. https://doi.org/10.1039/D3TB01723K.
Fittolani G, Vargová D, Seeberger PH, Ogawa Y, Delbianco M. Bottom-up approach to understand chirality transfer across scales in cellulose assemblies. J Am Chem Soc. 2022;144:12469–75. https://doi.org/10.1021/jacs.2c04522.
Hribernik N, Vargová D, Dal Colle MCS, Lim JH, Fittolani G, Yu Y, et al. Controlling the assembly of cellulose-based oligosaccharides through sequence modifications. Angew Chem Int Ed. 2023;62:e202310357. https://doi.org/10.1002/anie.202310357.
Langan P, Nishiyama Y, Chanzy H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules. 2001;2:410–6. https://doi.org/10.1021/bm005612q.
Buleon A, Chanzy H. Single crystals of cellulose II. J Polym Sci Polym Phys Ed. 1978;16:833–9. https://doi.org/10.1002/pol.1978.180160508.
Hata Y, Sawada T, Serizawa T. Macromolecular crowding for materials-directed controlled self-assembly. J Mater Chem B. 2018;6:6344–59. https://doi.org/10.1039/C8TB02201A.
Zhong C, Zajki-Zechmeister K, Nidetzky B. Effect of ionic liquid on the enzymatic synthesis of cello-oligosaccharides and their assembly into cellulose materials. Carbohydr Polym. 2023;301:120302. https://doi.org/10.1016/j.carbpol.2022.120302.
Serizawa T, Fukaya Y, Sawada T. Nanoribbon network formation of enzymatically synthesized cellulose oligomers through dispersion stabilization of precursor particles. Polym J. 2018;50:799–804. https://doi.org/10.1038/s41428-018-0057-3.
Kuga T, Sunagawa N, Igarashi K. Enzymatic synthesis of cellulose in space: gravity is a crucial factor for building cellulose II gel structure. Cellulose. 2022;29:2999–3015. https://doi.org/10.1007/s10570-021-04399-0.
Nohara T, Sawada T, Tanaka H, Serizawa T. Enzymatic synthesis of oligo(ethylene glycol)-bearing cellulose oligomers for in situ formation of hydrogels with crystalline nanoribbon network structures. Langmuir. 2016;32:12520–6. https://doi.org/10.1021/acs.langmuir.6b01635.
Sugiura K, Saito M, Sawada T, Tanaka H, Serizawa T. Cellodextrin phosphorylase-catalyzed single-process production and superior mechanical properties of organic-inorganic hybrid hydrogels composed of surface-carboxylated synthetic nanocelluloses and hydroxyapatite. ACS Sustain Chem Eng. 2022;10:13484–94. https://doi.org/10.1021/acssuschemeng.2c04349.
Serizawa T, Fukaya Y, Sawada T. Self-assembly of cellulose oligomers into nanoribbon network structures based on kinetic control of enzymatic oligomerization. Langmuir. 2017;33:13415–22. https://doi.org/10.1021/acs.langmuir.7b03653.
Tashiro M, Hata Y, Sawada T, Marubayashi H, Kawamura I, Serizawa T. Modulating neutralization-induced self-assembly of cello-oligosaccharides by organic solvents and temperature for preparing gels with improved mechanical properties. Cellulose. 2024;31:9057–73. https://doi.org/10.1007/s10570-024-06068-4.
Hanamura M, Sawada T, Serizawa T. In-paper self-assembly of cellulose oligomers for the preparation of all-cellulose functional paper. ACS Sustain Chem Eng. 2021;9:5684–92. https://doi.org/10.1021/acssuschemeng.1c00815.
Mizuuchi Y, Hata Y, Sawada T, Serizawa T. Surface-mediated self-assembly of click-reactive cello-oligosaccharides for fabricating functional nonwoven fabrics. Sci Technol Adv Mater. 2024;25:2311052. https://doi.org/10.1080/14686996.2024.2311052.
Linklater DP, Baulin VA, Juodkazis S, Crawford RJ, Stoodley P, Ivanova EP. Mechano-bactericidal actions of nanostructured surfaces. Nat Rev Microbiol. 2021;19:8–22. https://doi.org/10.1038/s41579-020-0414-z.
Hawi S, Goel S, Kumar V, Pearce O, Ayre WN, Ivanova EP. Critical review of nanopillar-based mechanobactericidal systems. ACS Appl Nano Mater. 2022;5:1–17. https://doi.org/10.1021/acsanm.1c03045.
Ivanova EP, Hasan J, Webb HK, Truong VK, Watson GS, Watson JA, et al. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small. 2012;8:2489–94. https://doi.org/10.1002/smll.201200528.
Anselme K, Davidson P, Popa AM, Giazzon M, Liley M, Ploux L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010;6:3824–46. https://doi.org/10.1016/j.actbio.2010.04.001.
Hasan J, Chatterjee K. Recent advances in engineering topography mediated antibacterial surfaces. Nanoscale. 2015;7:15568–75. https://doi.org/10.1039/C5NR04156B.
Mahanta U, Khandelwal M, Deshpande AS. Antimicrobial surfaces: a review of synthetic approaches, applicability and outlook. J Mater Sci. 2021;56:17915–41. https://doi.org/10.1007/s10853-021-06404-0.
Masuda T. Design of functional soft interfaces with precise control of the polymer architecture. Polym J. 2024;56:643–52. https://doi.org/10.1038/s41428-024-00908-7.
Visalakshan RM, Bright R, Burzava ALS, Barker AJ, Simon J, Ninan N, et al. Antibacterial nanostructured surfaces modulate protein adsorption, inflammatory responses, and fibrous capsule formation. ACS Appl Mater Interfaces. 2023;15:220–35. https://doi.org/10.1021/acsami.2c13415.
Martins de Sousa K, Linklater DP, Baulin VA, Dekiwadia C, Mayes E, Murdoch BJ, et al. Understanding the influence of serum proteins adsorption on the mechano-bactericidal efficacy and immunomodulation of nanostructured titanium. Adv Mater Interfaces. 2024;11:2301021. https://doi.org/10.1002/admi.202301021.
Acknowledgements
I sincerely thank Professor Takeshi Serizawa (Institute of Science Tokyo) and Professor Shingo Nakamura (National Defense Medical College) for their support and insightful discussions throughout this research. I am deeply grateful to Professor Toshiki Sawada (Institute of Science Tokyo), Professor Takamasa Sakai (University of Tokyo), Professor Hironori Marubayashi (Kyoto Institute of Technology), Professor Hiromi Miyazaki (National Defense Medical College), Professor Hidetoshi Matsumoto (Institute of Science Tokyo), Professor Shuichi Nojima (Tokyo Institute of Technology), Mr. Masahito Nishiura (DKS Co. Ltd.), and all the other collaborators for their invaluable contributions. This work was partially supported by Grants-in-Aid for Early Career Scientists (JP24K17732) from the Japan Society for the Promotion of Science and the Environment Research and Technology Development Fund (JPMEERF20241RA1) of the Environmental Restoration and Conservation Agency provided by the Ministry of the Environment of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hata, Y. Self-assembly of low-molecular-weight cellulose into nanostructured macroscopic materials. Polym J 58, 31–41 (2026). https://doi.org/10.1038/s41428-025-01101-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01101-0