Abstract
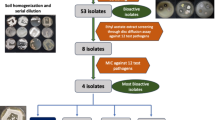
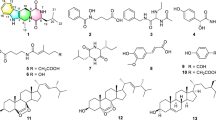
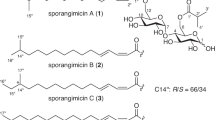
Bio-guided fractionation of the culture broth extract of Streptomyces asenjonii strain KNN 42.f recovered from an extreme hyper-arid Atacama Desert soil in northern Chile led to the isolation of three new bioactive β-diketones; asenjonamides A–C (1–3) in addition to the known N-(2-(1H-indol-3-yl)-2-oxoethyl)acetamide (4), a series of bioactive acylated 4-aminoheptosyl-β-N-glycosides; spicamycins A–E (5–9), and seven known diketopiperazines (10–16). All isolated compounds were characterized by HRESIMS and NMR analyses and tested for their antibacterial effect against a panel of bacteria.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Pidot SJ, Coyne S, Kloss F, Hertweck C. Antibiotics from neglected bacterial sources. Int J Med Microbiol. 2014;304:14–22.
Horikoshi K, Antranikian G, Bull AT, Robb FT, Stetter KO, editors. Extremophiles Handbook. Tokyo: Springer; 2011.
Guo X, Liu N, Li X, Ding Y, Shang F, Gao Y, Ruan J, Huang Y. Red soils harbor diverse culturable actinomycetes that are promising sources of novel secondary metabolites. Appl Environ Microbiol. 2015;81:3086–103.
Gómez-Silva B, Rainey FA, Warren-Rhodes KA, McKay CP, Navarro-González R. Atacama Desert soil microbiology. In: Dion P, Nautiyal CS, editors. Microbiology of Extreme Soils, Soil Biology. Berlin: Springer; 2008. Vol. 13, pp. 117−132.
Navarro-González R, et al. Mars-Like soils in the Atacama Desert, Chile, and the dry limit of microbial Life. Science. 2003;302:1018–21.
Crits-Christoph A, et al. Colonization patterns of soil microbial communities in the Atacama Desert. Microbiome. 2013;1:28 https://doi.org/10.1186/2049-2618-1-28.
Bull AT, Asenjo JA, Goodfellow M, Go´mez-Silva B. The Atacama Desert: technical resources and the growing importance of novel microbial diversity. Ann Rev Microbiol. 2016;70:215–34.
Goodfellow M, Fiedler HP. A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie Van Leeuwenhoek. 2010;98:119–42.
Wichner D, et al. Isolation and anti-HIV-1 integrase activity of lentzeosides A–F from extremotolerant lentzea sp. H45, a strain isolated from a high-altitude Atacama Desert soil. J Antibiot. 2017;70:448–53.
Rateb ME, et al. Chaxamycins A-D, bioactive ansamycins from a hyper-arid desert Streptomyces sp. J Nat Prod. 2011;74:1491–99.
Rateb ME, et al. Diverse metabolic profiles of a Streptomyces strain isolated from a hyper-arid environment. J Nat Prod. 2011;74:1965–71.
Busarakam K, et al. Streptomyces leeuwenhoekii sp. nov., the producer of chaxalactins and chaxamycins, forms a distinct branch in Streptomyces gene trees. Antonie Van Leeuwenhoek. 2014;105:849–61.
Schulz D, et al. Abenquines A–D: aminoquinone derivatives produced by Streptomyces sp. strain DB634. J Antibiot. 2011;64:763–8.
Nachtigall J, et al. Atacamycins A–C, 22-membered antitumor macrolactones produced by Streptomyces sp. C38. J Antibiot. 2011;64:775–80.
Elsayed SS, et al. Chaxapeptin, a lasso peptide from extremotolerant Streptomyces leeuwenhoekii strain C58 from the hyper-arid Atacama Desert. J Org Chem. 2015;80:10252–60.
Wakefield J, Hassan HM, Jaspars M, Ebel R, Rateb ME. Dual induction of new microbial secondary metabolites by fungal bacterial co-cultivation. Front Microbiol. 2017;8:1284 https://doi.org/10.3389/fmicb.2017.01284.
Norte M, Cataldo F, González AG. Siphonarienedione and siphonarienolone, two new metabolites from Siphonaria grisea having a polypropionate skeleton. Tetrahedron Lett. 1988;29:2879–80.
Calter MA, Liao W. First total synthesis of a natural product containing a chiral, β-diketone: Synthesis and stereochemical reassignment of siphonarienedione and siphonarienolone. J Am Chem Soc. 2002;124:13127–9.
Iakovou K, et al. Design, synthesis and biological evaluation of novel b-substituted indol-3-yl ethylamido melatoninergic analogues. J Pharm Pharmacol. 2002;54:147–56.
Jian Y, Nan W, Hai-Sheng Y, Jiang-Chun H, Yu-Cheng D. A new Sesquiterpene from the medicinal fungus Inonotus vaninii. Chem Nat Comp. 2013;49:261–3.
Hayakawa Y, et al. Studies on the differentiation inducers of myeloid leukemic cells III. Spicamycin, a new inducer of differentiation of HL-60 human promyelocytic leukemia cells. J Antibiot. 1983;36:934–7.
Suzuki T, Suzuki ST, Yamada I, Koashi Y, Yamada K, Chida N. Total synthesis of spicamycin. J Org Chem. 2002;67:2874–80.
Hayakawa Y, et al. Spicamycin, a new differentiation inducer of mouse myeloid leukemia cells Ml and human promyelocytic leukemia cells HL-60. Agric Biol Chem. 1985;49:2685–91.
Chen M, Dewis K, Kraut K, Merritt D, Reiber L, Trinnaman L, Da Costa N. 2,5-Diketopiperazines (Cyclic Dipeptides) in Beef: Identification, synthesis, and sensory evaluation. J Food Sci. 2009;74:100–5.
Amira R, Yoel K, Yehuda B, Michael S. Amino acid derivatives from the marine sponge Jaspis digonoxea. J Nat Prod. 1994;57:829–32.
Muhanna M, Juriyati J, Nik M, Ruangelie E, Noraziah M. Isolation and characterization of cyclo-(tryptophanyl-prolyl) and chloramphenicol from Streptomyces sp. SUK 25 with antimethicillin-resistant S. aureus activity. Drug Des Dev Ther. 2016;10:1817–27.
Bin L, Gang C, Jiao B, Yong-Kui J, Yue-Hu P. A bisamide and four diketopiperazines from a marine-derived Streptomyces sp. J Asian Nat Prod Res. 2011;13:1146–50.
Ström K, Sjögren J, Broberg A, Schnürer J. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid. Appl Environ Microbiol. 2002;68:4322–7.
Mitova M, Giuseppina T, Ute H, Mueller WEG, Salvatore D-R. Exocellular cyclic dipeptides from a Ruegeria strain associated with cell cultures of Suberites domuncula. Mar Biotechnol. 2004;6:95–103.
Goodfellow M, et al. Streptomyces asenjonii sp. nov., isolated from hyper-arid Atacama Desert soils and emended description of Streptomyces viridosporus Pridham et al. 1958. Antonie Van Leeuwenhoek. 2017;110:1133–48.
Idris H, Goodfellow M, Sanderson R, Asenjo JA, Bull. AT. Actinobacterial rare biospheres and dark matter revealed in habitats of the Chilean Atacama Desert. Sci Rep. 2017;7:8373 https://doi.org/10.1038/s41598-017-08937-4.
Silber J, Ohlendorf B, Labes A, Näther C, Imhoff JF. Calcaripeptides A–C. cyclodepsipeptides from a Calcarisporium Strain. J Nat Prod. 2013;76:1461–7.
Plaza A, et al. Aetheramides A and B, potent HIV-Inhibitory depsipeptides from a Myxobacterium of the new Genus “Aetherobacter”. Org Lett. 2012;14:2854–7.
Rateb ME, et al. Induction of diverse secondary metabolites in Aspergillus fumigatus by microbial co-culture. RSC Adv. 2013;3:14444–50.
Zakharova OS, Zenova GM, Zvyagintsey DG. Some approaches to the selective isolation of actinomycetes of the genus Actinomadura from soil. Microbiology. 2003;72:110–3.
Zhang D, Noviendri D, Nursid M, Yang X, Son BW. 12,13-dihydroxyfumitremorgin C, fumitremorgin C, and brevianamide F, antibacterial diketopiperazine alkaloids from the marine-derived fungus Pseudallescheria sp. Nat Prod Sci. 2007;13:251–4.
Kronvall G. Single-strain regression analysis for determination of interpretive breakpoints for cefoperazone disk diffusion susceptibility testing. J Clin Microbiol. 1983;17:975–80.
Acknowledgements
We thank the College of Physical Sciences, University of Aberdeen, for provision of infrastructure and facilities in the Marine Biodiscovery Centre. This work was supported financially by the Biotechnological and Biological Sciences Research Council under the RCUK-CONICYT Newton International Research Project (JIC: CA586) and the Basal Programme of CONICYT for funding of the Centre for Biotechnology and Bioengineering, CeBiB (project FB0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Abdelkader, M.S.A., Philippon, T., Asenjo, J.A. et al. Asenjonamides A–C, antibacterial metabolites isolated from Streptomyces asenjonii strain KNN 42.f from an extreme-hyper arid Atacama Desert soil. J Antibiot 71, 425–431 (2018). https://doi.org/10.1038/s41429-017-0012-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41429-017-0012-0
This article is cited by
-
The Blessed Clay from Boho, Northern Ireland: Can the Nature of Spiritual Healing Sites Guide us Towards New Sources of Drug Discovery?
Journal of Religion and Health (2025)
-
Going to extremes: progress in exploring new environments for novel antibiotics
npj Antimicrobials and Resistance (2024)
-
Mining microbial and metabolic dark matter in extreme environments: a roadmap for harnessing the power of multi-omics data
Advanced Biotechnology (2024)
-
Polyphasic characterization and genomic insights into Nocardioides turkmenicus sp. nov. isolated from a desert soil
Antonie van Leeuwenhoek (2024)
-
Bioprospecting of desert actinobacteria with special emphases on griseoviridin, mitomycin C and a new bacterial metabolite producing Streptomyces sp. PU-KB10–4
BMC Microbiology (2023)