Abstract
Background/Objectives
Sickle cell anemia (SCA) is marked by hypoxia, inflammation, and secondary iron overload (IO), which potentially modulate hepcidin, the pivotal hormone governing iron homeostasis. The aim was to evaluate the iron incorporation in red blood cells (RBC) in SCA pediatric patients, considering the presence or absence of IO.
Subjects/Methods
SCA children (n = 12; SCAtotal) ingested an oral stable iron isotope (57Fe) and iron incorporation in RBC was measured after 14 days. Patients with ≥1000 ng/mL serum ferritin were considered to present IO (SCAio+; n = 4) while the others were classified as being without IO (SCAio−; n = 8). Liver iron concentration (LIC) was determined by Magnetic Resonance Imaging (MRI) T2* method.
Results
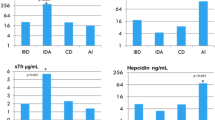
The SCAio+ group had lower iron incorporation (mean ± SD: 0.166 ± 0.04 mg; 3.33 ± 0.757%) than SCAio− patients (0.746 ± 0.303 mg; 14.9 ± 6.05%) (p = 0.024). Hepcidin was not different between groups. Iron incorporation was inversely associated with serum ferritin level (SCAtotal group: r = −0.775, p = 0.041; SCAio− group: r = −0.982; p = 0.018) and sickle hemoglobin (HbS) presented positive correlation with iron incorporation (r = 0.991; p = 0.009) in SCAio− group. LIC was positively associated with ferritin (SCAtotal: r = 0.921; p = 0.026) and C reactive protein (SCAio+: r = 0.999; p = 0.020).
Conclusion
SCAio+ group had lower iron incorporation in RBC than SCAio− group, suggesting that they may not need to reduce their intake of iron-rich food, as usually recommended. Conversely, a high percentage of HbS may indirectly exacerbate hypoxia and seems to increase iron incorporation in RBC.
Trial Registration
This trial was registered at www.ensaiosclinicos.gov.br. Identifier RBR-4b7v8pt.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data analyzed in this study are available from the corresponding author on reasonable request.
References
Inusa BPD, Hsu LL, Kohli N, Patel A, Ominu-Evbota K, Anie KA, et al. Sickle cell disease – genetics, pathophysiology, clinical presentation and treatment. Int J Neonatal Screen. 2019;5:20 https://doi.org/10.3390/ijns5020020.
Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–31. https://doi.org/10.1016/S0140-6736(10)61029-X.
Nascimento MI, Przibilski ALF, Coelho CSG, Leite KFA, Makenze M, Jesus SB. Mortality attributed to sickle cell disease in children and adolescents in Brazil, 2000-2019. Rev Saude Publica. 2022;56:65 https://doi.org/10.11606/s1518-8787.2022056003681.
Lobo CLC, Nascimento EMD, Jesus LJC, Freitas TG, Lugon JR, Ballas SK. Mortality in children, adolescents and adults with sickle cell anemia in Rio de Janeiro, Brazil. Rev Bras Hematol Hemoter. 2018;40:37–42. https://doi.org/10.1016/j.bjhh.2017.09.006.
Inati A, Khoriaty E, Mussalam KM. Iron sickle-cell disease: what have we learned over the years? Pediatr Blood Cancer. 2011;56:182–90. https://doi.org/10.1002/pbc.22721.
Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus Med Rev. 2007;21:118–33. https://doi.org/10.1016/j.tmrv.2006.11.003.
Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood. 2012;120:3657–69. https://doi.org/10.1182/blood-2012-05-370098.
Ohemeng A, Boadu I. The role of nutrition in the pathophysiology and management of sickle cell disease among children: a review of literature. Crit Rev Food Sci Nutr. 2018;58:2299–305. https://doi.org/10.1080/10408398.2017.1319794.
Teixeira TV, Da Silva ACF, Rodrigues CDSC, Brito FDSB, Canella DS, Citelli M. Food consumption of people with sickle cell anemia in a middle-income country. Nutrients. 2023;15:1478 https://doi.org/10.3390/nu15061478.
Omena J, Curioni C, Rodrigues CDSC, Citelli M. The effect of food and nutrients on iron overload: what do we know so far? Eur J Clin Nutr. 2021;75:1771–80. https://doi.org/10.1038/s41430-021-00887-5.
Black MM. Integrated strategies needed to prevent iron deficiency and to promote early child development. J Trace Elem Med Biol. 2012;26:120–3. https://doi.org/10.1016/j.jtemb.2012.04.020.
Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434–43. https://doi.org/10.1016/j.bbamcr.2012.01.014.
Omena J, Cople-Rodrigues CS, Cardoso JDA, Soares AR, Fleury MK, Brito FSB, et al. Serum hepcidin concentration in individuals with sickle cell anemia: Basis for the dietary recommendation of iron. Nutrients. 2018;10:498 https://doi.org/10.3390/nu10040498.
Mangaonkar AA, Thawer F, Son J, Ajebo G, Xu H, Barrett NJ, et al. Regulation of iron homeostasis through the erythroferrone-hepcidin axis in sickle cell disease. Br J Haematol. 2020;189:1204–9. https://doi.org/10.1111/bjh.16498.
Erlandson ME, Walden B, Stern G, Hilgartner MW, Wehman J, Smith CH. Sudies on congenital hemolytic syndromes, IV. Gastrointestinal absorption of iron. Blood. 1962;19:359–78.
Sarria B, Dainty JR. Comparison of faecal monitoring and area under the curve techniques to determine iron absorption in humans using stable isotope labelling. J Trace Elem Med Biol. 2010;24(3):157–60. https://doi.org/10.1016/j.jtemb.2010.01.010.
Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematol Am Soc Hematol Educ Program. 2013;2013:447–56. https://doi.org/10.1182/asheducation-2013.1.447.
Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D, et al. Liver iron quantification with MR imaging: a primer for radiologists. Radiographics. 2018;38:392–412. https://doi.org/10.1148/rg.2018170079.
Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N. Engl J Med. 2000;343:327–31. https://doi.org/10.1056/NEJM200008033430503.
St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–61. https://doi.org/10.1182/blood-2004-01-0177.
Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, et al. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–5. https://doi.org/10.1182/blood-2008-12-191643.
Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, et al. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357–62. https://doi.org/10.1016/S0140-6736(04)15436-6.
International Atomic Energy Agency (IAEA). Assessment of iron bioavailability in humans using stable iron isotope techniques. IAEA Human Health Series. Vienna: International Atomic Energy Agency; 2012.
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr. 1994;71:411–24. https://doi.org/10.1079/bjn19940148.
Chen Z, Griffin IJ, Plumlee LM, Abrams SA. High resolution inductively coupled plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr. 2005;135:1790–5. https://doi.org/10.1093/jn/135.7.1790.
Braz BF, Omena J, Voll VM, Citelli M, Rodrigues CDSC, Cincotto FH, et al. Novel bioanalytical strategy using isotope pattern deconvolution and ICP-QMS for the study of iron incorporation in erythrocytes: An insight to better assessment. Talanta. 2024;270:125579 https://doi.org/10.1016/j.talanta.2023.125579.
Riley AA, Arakawa Y, Worley S, Duncan BW, Fukamachi K. Circulating blood volumes: a review of measurement techniques and a meta-analysis in children. ASAIO J. 2010;56:260–4. https://doi.org/10.1097/MAT.0b013e3181d0c28d.
Lobo CLC, Ballas SK, Domingos ACB, Moura PG, Nascimento EM, Cardoso GP, et al. Newborn screening program for hemoglobinopathies in Rio de Janeiro, Brazil. Pediatr Blood Cancer. 2014;61:34–9. https://doi.org/10.1002/pbc.24711.
World Health Organization. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee. Technical Report Series (854); Geneva: World Health Organization; 1995.
Kearney SL, Nemeth E, Neufeld EJ, Thapa D, Ganz T, Weinstein DA, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. https://doi.org/10.1002/pbc.20616.
Lynch SR, Skikne BS, Cook JD. Food iron absorption in idiopathic hemochromatosis. Blood. 1989;74:2187–93.
Cook JD, Lipschitz DA, Miles LE, Finch CA. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr. 1974;27:681–7. https://doi.org/10.1093/ajcn/27.7.681.
Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, et al. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119:1922–8. https://doi.org/10.1182/blood-2011-11-391219.
Jonker FAM, Calis JCJ, Phiri K, Kraaijenhagen RJ, Brabin BJ, Faragher B, et al. Low Hepcidin Levels in Severely Anemic Malawian Children with High Incidence of Infectious Diseases and Bone Marrow Iron Deficiency. PLoS One. 2013;8:e78964 https://doi.org/10.1371/journal.pone.0078964.
Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr. 2009;90:1280–7. https://doi.org/10.3945/ajcn.2009.28129.
Aksan A, Wohlrath M, Iqbal TH, Farrag K, Dignass A, Stein J. Serum Hepcidin Levels Predict Intestinal Iron Absorption in Patients with Inflammatory Bowel Disease. Clin Lab 2019;65. https://doi.org/10.7754/Clin.Lab.2019.190106.
Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–41. https://doi.org/10.1152/physrev.00008.2013.
Tancabelic J, Sheth S, Paik M, Piomelli S. Serum transferrin receptor as a marker of erythropoiesis suppression in patients on chronic transfusion. Am J Hematol. 1999;60:121–5. https://doi.org/10.1002/(sici)1096-8652(199902)60<121::aid-ajh6>23.0.co;2-2.
Grote Beverborg N, Verweij N, Klip IT, van der Wal HH, Voors AA, van Veldhuisen DJ, et al. PLoS One. 2015;10:e0125215 https://doi.org/10.1371/journal.pone.0125215.
Pressac M, Morgant G, Farnier MA, Aymard P. Enzyme immunoassay of serum erythropoietin in healthy children: reference values. Ann Clin Biochem. 1991;28:345–50. https://doi.org/10.1177/000456329102800405.
Acknowledgements
We would like to thank all the volunteers who participated in this study and the excellent technical assistance provided by Isis Rodrigues, Viviane F. Meneses, Clarice M. Carvalho, Valdilene L. Souza, Elizabeth Pereira and Verônica Barbosa.
Funding
This study was funded by the Ministry of Health (Grant no. 777022/2012); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant no. 408401/2017-6); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance Code 001); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Grant no. E-26-010.100930/2018 and E-26/200.963/2022).
Author information
Authors and Affiliations
Contributions
MC, JO, FFB, and CSCR designed the study; CMD contributed to the design of the work; MC and CSCR were responsible for obtaining funding; JO, MC, and VMV conducted the research; JO, VMV, MC, BFB and RES conducted the laboratory analysis; FFB and CMD helped to interpret the data and provided critical suggestions and comments; GFJ and ASR conducted the MRI procedures; JO, VMV, FFB, and MC performed the statistical analysis; JO, VMV, and MC wrote the manuscript, and had primary responsibility for the final content. All authors read, contributed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research was performed in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of State Institute of Hematology Arthur de Siqueira Cavalcanti (419/17; 2.788.659) and Pedro Ernesto University Hospital (2.695.418). The study was conducted only on children whose parents or guardians agreed to their participation and signed a free and informed consent form. The study was registered at www.ensaiosclinicos.gov.br (Identifier RBR-4b7v8pt).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omena, J., Voll, V.M., Bezerra, F.F. et al. Iron incorporation in red blood cells of pediatric sickle cell anemia: a stable isotope pilot investigation. Eur J Clin Nutr 78, 801–807 (2024). https://doi.org/10.1038/s41430-024-01465-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01465-1