Abstract
Background
Deficiency in vitamin D is widely prevalent around the world. Oral vitamin D supplementation is suggested for older adults to sustain appropriate 25-hydroxyvitamin D (25(OH)D) levels throughout the year. At present, cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2) are the most commonly used compounds. Supplementation with calcifediol (25OHD3) rather than vitamin D itself should also be considered for the treatment. We performed a systematic review of the literature with a meta-analysis to assess the effects of cholecalciferol (vitamin D3) compared to calcifediol (25OHD3) on increasing serum 25(OH)D levels.
Methods
A search of online databases was performed electronically for all relevant observational published population-based studies until November 2023, without geographical restrictions. We included studies that directly compared the effects of cholecalciferol and calcifediol on increasing concentrations of serum 25(OH)D. Only papers in English or French languages were considered. Records were screened and data were retrieved through a standardized extraction process.
Results
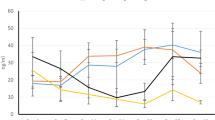
Seventeen studies including 1575 participants were reviewed. Twelve intervention trials showed that, in spite of the dosage or the frequency of administration, calcifediol supplementation was more efficacious in raising serum 25(OH)D concentrations compared with cholecalciferol. Two studies showed that calcifediol and cholecalciferol were identically potent. According to three studies, cholecalciferol was more effective than calcifediol in raising 25(OH)D concentrations. A meta-analysis including randomized controlled trials (RCTs) and non-randomized trials revealed that calcifediol supplementation had a better impact on elevating serum 25(OH)D concentrations compared with the effect of cholecalciferol.
Conclusion
This meta-analysis suggests that calcifediol is more effective in increasing serum 25(OH)D concentrations compared to cholecalciferol. Consequently, calcifediol may emerge as the preferred option for supplementation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev. 2012;33:456–92.
Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:2097.
Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2018;40:1109–51.
Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45.
Dominguez LJ, Farruggia M, Veronese N, Barbagallo M. Vitamin D sources, metabolism, and deficiency: available compounds and guidelines for its treatment. Metabolites. 2021;11:255.
Gilchrest BA. Sun protection and Vitamin D: three dimensions of obfuscation. J Steroid Biochem Mol Biol. 2007;103:655–63.
Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29:1697–711.
Pérez-Castrillón JL, Dueñas-Laita A, Brandi ML, Jódar E, Del Pino-Montes J, Quesada-Gómez JM, et al. Calcifediol is superior to cholecalciferol in improving vitamin D status in postmenopausal women: a randomized trial. J Bone Min Res. 2021;36:1967–78.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Navarro-Valverde C, Sosa-Henríquez M, Alhambra-Expósito MR, Quesada-Gómez JM. Vitamin D3 and calcidiol are not equipotent. J Steroid Biochem Mol Biol. 2016;164:205–8.
Rossini M, Viapiana O, Gatti D, James G, Girardello S, Adami S. [The long term correction of vitamin D deficiency: comparison between different treatments with vitamin D in clinical practice.]. Minerva Med. 2005;96:1–7.
Meyer O, Dawson-Hughes B, Sidelnikov E, Egli A, Grob D, Staehelin HB, et al. Calcifediol versus vitamin D3 effects on gait speed and trunk sway in young postmenopausal women: a double-blind randomized controlled trial. Osteoporos Int. 2015;26:373–81.
Stamp TC, Haddad JG, Twigg CA. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet. 1977;1:1341–3.
Shieh A, Lee SM, Lagishetty V, Gottleib C, Jacobs JP, Adams JS. Pilot trial of vitamin D3 and calcifediol in healthy vitamin D deficient adults: does it change the fecal microbiome? J Clin Endocrinol Metab. 2021;106:3464–76.
Cashman KD, Seamans KM, Lucey AJ, Stöcklin E, Weber P, Kiely M, et al. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95:1350–6.
Graeff-Armas LA, Bendik I, Kunz I, Schoop R, Hull S, Beck M. Supplemental 25-hydroxycholecalciferol is more effective than cholecalciferol in raising serum 25-hydroxyvitamin D concentrations in older adults. J Nutr 2020;150:73–81.
Charoenngam N, Kalajian TA, Shirvani A, Yoon GH, Desai S, McCarthy A, et al. A pilot-randomized, double-blind crossover trial to evaluate the pharmacokinetics of orally administered 25-hydroxyvitamin D3 and vitamin D3 in healthy adults with differing BMI and in adults with intestinal malabsorption. Am J Clin Nutr. 2021;114:1189–99.
Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8:222–30.
Shieh A, Ma C, Chun RF, Witzel S, Rafison B, Contreras HTM, et al. Effects of cholecalciferol vs calcifediol on total and free 25-hydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2017;102:1133–40.
Vaes AMM, Tieland M, de Regt MF, Wittwer J, van Loon LJC, de Groot LCPGM. Dose-response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37:808–14.
Corrado A, Rotondo C, Cici D, Berardi S, Cantatore FP. Effects of different vitamin D supplementation schemes in post-menopausal women: a monocentric open-label randomized study. Nutrients. 2021;13:380.
Ruggiero C, Baroni M, Bini V, Brozzetti A, Parretti L, Zengarini E, et al. Effects of weekly supplementation of cholecalciferol and calcifediol among the oldest-old people: findings from a randomized pragmatic clinical trial. Nutrients. 2019;11:2778.
Di Nisio A, De Toni L, Sabovic I, Rocca MS, De Filippis V, Opocher G, et al. Impaired release of vitamin D in dysfunctional adipose tissue: new cues on vitamin D supplementation in obesity. J Clin Endocrinol Metab. 2017;102:2564–74.
Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Edel JO, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Min Res. 2012;27:160–9.
Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoecklin E, Goessl R, et al. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone. 2014;59:14–9.
Stamp TC. Intestinal absorption of 25-hydroxycholecalciferol. Lancet. 1974;2:121–3.
Heaney RP, Horst RL, Cullen DM, Armas LAG. Vitamin D3 distribution and status in the body. J Am Coll Nutr. 2009;28:252–6.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408.
Sitrin MD, Pollack KL, Bolt MJ, Rosenberg IH. Comparison of vitamin D and 25-hydroxyvitamin D absorption in the rat. Am J Physiol. 1982;242:G326–32.
Bouillon R. Genetic and racial differences in the vitamin D endocrine system. Endocrinol Metab Clin North Am. 2017;46:1119–35.
Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101:7711–5.
Elkum N, Alkayal F, Noronha F, Ali MM, Melhem M, Al-Arouj M, et al. Vitamin D insufficiency in Arabs and South Asians positively associates with polymorphisms in GC and CYP2R1 genes. PLoS ONE. 2014;9:e113102.
Author information
Authors and Affiliations
Contributions
O. Saidane and L. Rouached were responsible for data collection. S. Bouden and M. Ben Messaoud contributed to the manuscript writing. C. Dziri performed the statistical analysis. A. Ben Tekaya and I. Mahmoud critically revised the manuscript for intellectual content. R. Tekaya and L. Abdelmoula supervised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouden, S., Ben Messaoud, M., Saidane, O. et al. Effect of cholecalciferol versus calcifediol on serum 25(OH)D concentrations: a systematic review with meta-analysis. Eur J Clin Nutr 79, 296–305 (2025). https://doi.org/10.1038/s41430-024-01520-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01520-x