Abstract
Background/objectives
Serum total antioxidant capacity (sTAC) and uric acid (SUA) levels have been related to oxidative stress in metabolic diseases. Nevertheless, the evidence in epidemiological studies is still scarce and inconsistent. We aimed to evaluate the association between sTAC, SUA, obesity, and cardiometabolic traits in Mexican children.
Subjects/methods
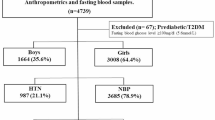
This cross-sectional study analyzed anthropometric data, blood pressure, cardiometabolic traits, and SUA levels of 248 children with normal weight (NW) and 255 with obesity (OB). sTAC was measured with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method.
Results
sTAC was directly related to SUA (β = 0.905 ± 0.358, p = 0.012). Obesity was positively associated with sTAC (β = 0.075 ± 0.020, p < 0.001) and SUA (β = 0.706 ± 0.129, p < 0.001). sTAC was negatively associated with diastolic blood pressure (β = -8.458 ± 3.758, p = 0.026) in NW children and positively associated with insulin (β = 9.511 ± 3.107, p = 0.002) and the homeostatic model assessment of insulin resistance (β = 2.065 ± 0.680, p = 0.003) in OB children. SUA showed negative associations with total cholesterol (β = -4.062 ± 1.340, p = 0.003) and low-density lipoprotein cholesterol (β = -2.470 ± 1.190, p = 0.039) in NW children and high-density lipoprotein cholesterol (β = -1.306 ± 0.409, p < 0.01) in OB children.
Conclusion
sTAC and SUA are positively associated and are increased in obesity. According to weight status, sTAC and SUA are associated with blood pressure, insulin resistance markers, and lipid profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Shamah-Levy T, Gaona-Pineda EB, Cuevas-Nasu L, Valenzuela-Bravo DG, Morales-Ruan C, Rodríguez-Ramírez S, et al. Sobrepeso y obesidad en población escolar y adolescente. salud pública de méxico. 2024;66:404–13.
Vahid F, Rahmani D, Davoodi SH. The correlation between serum inflammatory, antioxidant, glucose handling biomarkers, and Dietary Antioxidant Index (DAI) and the role of DAI in obesity/overweight causation: population-based case-control study. Int J Obes (Lond). 2021;45:2591–9.
Talegawkar SA, Beretta G, Yeum KJ, Johnson EJ, Carithers TC, Taylor HA Jr, et al. Total antioxidant performance is associated with diet and serum antioxidants in participants of the diet and physical activity substudy of the Jackson Heart Study. J Nutr. 2009;139:1964–71.
Farhangi MA, Vajdi M, Fathollahi P. Dietary total antioxidant capacity (TAC), general and central obesity indices and serum lipids among adults: An updated systematic review and meta-analysis. Int J Vitam Nutr Res. 2022;92:406–22.
Puchau B, Ochoa MC, Zulet MA, Marti A, Martinez JA, Members G. Dietary total antioxidant capacity and obesity in children and adolescents. Int J Food Sci Nutr. 2010;61:713–21.
Kilic E, Ozer OF, Erek Toprak A, Erman H, Torun E, Kesgin Ayhan S, et al. Oxidative stress status in childhood obesity: a potential risk predictor. Med Sci Monit. 2016;22:3673–9.
Jaksic M, Martinovic M, Gligorovic-Barhanovic N, Vujacic A, Djurovic D, Nedovic-Vukovic M. Association between inflammation, oxidative stress, vitamin D, copper and zinc with pre-obesity and obesity in school children from the city of Podgorica, Montenegro. J Pediatr Endocrinol Metab. 2019;32:951–7.
Rowicka G, Dylag H, Ambroszkiewicz J, Riahi A, Weker H, Chelchowska M. Total oxidant and antioxidant status in prepubertal children with obesity. Oxid Med Cell Longev. 2017;2017:5621989.
Vehapoglu A, Turkmen S, Goknar N, Ozer OF. Reduced antioxidant capacity and increased subclinical inflammation markers in prepubescent obese children and their relationship with nutritional markers and metabolic parameters. Redox Rep. 2016;21:271–80.
Ruperez AI, Mesa MD, Anguita-Ruiz A, Gonzalez-Gil EM, Vazquez-Cobela R, Moreno LA, et al. Antioxidants and oxidative stress in children: influence of puberty and metabolically unhealthy status. Antioxidants (Basel). 2020;9:618–36.
Correia-Costa L, Sousa T, Morato M, Cosme D, Afonso J, Areias JC, et al. Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function. Br J Nutr. 2016;116:805–15.
Eren E, Abuhandan M, Solmaz A, Taskin A. Serum paraoxonase/arylesterase activity and oxidative stress status in children with metabolic syndrome. J Clin Res Pediatr Endocrinol. 2014;6:163–8.
Morandi A, Corradi M, Piona C, Fornari E, Puleo R, Maffeis C. Systemic anti-oxidant capacity is inversely correlated with systolic blood pressure and pulse pressure in children with obesity. Nutr Metab Cardiovasc Dis. 2020;30:508–13.
Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29:1106–14.
Wayner DD, Burton GW, Ingold KU, Barclay LR, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987;924:408–19.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6.
Thomazini F, de Carvalho BS, de Araujo PX, Franco MDC. High uric acid levels in overweight and obese children and their relationship with cardiometabolic risk factors: what is missing in this puzzle?. J Pediatr Endocrinol Metab. 2021;34:1435–41.
Albuja-Quintana N, Chisaguano-Tonato AM, Herrera-Fontana ME, Figueroa-Samaniego S, Alvarez-Suarez JM. Relationship between plasma uric acid levels, antioxidant capacity, and oxidative damage markers in overweight and obese adults: A cross-sectional study. PLoS One. 2025;20:e0312217.
Ye W, Zhou X, Xu Y, Zheng C, Liu P. Serum uric acid levels among chinese children: reference values and association with overweight/obesity. Clin Pediatr (Philos). 2024;63:1684–90.
Zheng R, Chen C, Yang T, Chen Q, Lu R, Mao Y. Serum uric acid levels and the risk of obesity: a longitudinal population-based epidemiological study. Clin Lab. 2017;63:1581–7.
Zeng J, Lawrence WR, Yang J, Tian J, Li C, Lian W, et al. Association between serum uric acid and obesity in Chinese adults: a 9-year longitudinal data analysis. BMJ Open. 2021;11:e041919.
Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Report. 2013:1–3.
Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30.
Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63:976–81.
Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis?. Atherosclerosis. 2000;148:131–9.
Wang Y, Yang M, Lee SG, Davis CG, Kenny A, Koo SI, et al. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J Nutr Biochem. 2012;23:1725–31.
Dominguez-Zambrano E, Pedraza-Chaverri J, Lopez-Santos AL, Medina-Campos ON, Cruz-Rivera C, Bueno-Hernandez F, et al. Association between serum uric acid levels, nutritional and antioxidant status in patients on hemodialysis. Nutrients. 2020;12:2600–13.
Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci Usa. 1981;78:6858–62.
Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93:284–9.
Anaya-Morua W, Villafan-Bernal JR, Ramirez-Moreno E, Garcia-Ortiz H, Martinez-Portilla RJ, Contreras-Cubas C, et al. Total antioxidant capacity in obese and non-obese subjects and its association with anthropo-metabolic markers: systematic review and meta-analysis. Antioxidants (Basel). 2023;12:1512–27.
Ortner Hadziabdic M, Vitali Cepo D, Rahelic D, Bozikov V. The effect of the mediterranean diet on serum total antioxidant capacity in obese patients: a randomized controlled trial. J Am Coll Nutr. 2016;35:224–35.
Lim SH, Fan SH, Say YH. Plasma total antioxidant capacity (TAC) in obese Malaysian subjects. Malays J Nutr. 2012;18:345–54.
Mohammadi S, Lotfi K, Mirzaei S, Asadi A, Akhlaghi M, Saneei P. Dietary total antioxidant capacity in relation to metabolic health status in overweight and obese adolescents. Nutr J. 2022;21:54.
Puchau B, Zulet MA, de Echavarri AG, Hermsdorff HH, Martinez JA. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition. 2010;26:534–41.
Carrion-Garcia CJ, Guerra-Hernandez EJ, Garcia-Villanova B, Molina-Montes E. Non-enzymatic antioxidant capacity (NEAC) estimated by two different dietary assessment methods and its relationship with NEAC plasma levels. Eur J Nutr. 2017;56:1561–76.
Svetkey LP, Simons-Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–93.
Bae JC, Seo SH, Hur KY, Kim JH, Lee MS, Lee MK, et al. Association between serum albumin, insulin resistance, and incident diabetes in nondiabetic subjects. Endocrinol Metab (Seoul). 2013;28:26–32.
Lloyd CE, Kalinyak JE, Hutson SM, Jefferson LS. Stimulation of albumin gene transcription by insulin in primary cultures of rat hepatocytes. Am J Physiol. 1987;252:C205–14.
Anraku M, Chuang VT, Maruyama T, Otagiri M. Redox properties of serum albumin. Biochim Biophys Acta. 2013;1830:5465–72.
Li X, Chen D, Wang G, Lu Y. Probing the interaction of human serum albumin with DPPH in the absence and presence of the eight antioxidants. Spectrochim Acta A Mol Biomol Spectrosc. 2015;137:1144–52.
Bravi MC, Armiento A, Laurenti O, Cassone-Faldetta M, De Luca O, Moretti A, et al. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2006;55:691–5.
Takahashi S, Hisatsune A, Kurauchi Y, Seki T, Katsuki H. Insulin-like growth factor 1 specifically up-regulates expression of modifier subunit of glutamate-cysteine ligase and enhances glutathione synthesis in SH-SY5Y cells. Eur J Pharmacol. 2016;771:99–106.
Nagao H, Nishizawa H, Tanaka Y, Fukata T, Mizushima T, Furuno M, et al. Hypoxanthine secretion from human adipose tissue and its increase in hypoxia. Obesity (Silver Spring). 2018;26:1168–78.
Lin WT, Chan TF, Huang HL, Lee CY, Tsai S, Wu PW, et al. Fructose-rich beverage intake and central adiposity, uric acid, and pediatric insulin resistance. J Pediatr. 2016;171:90–6.e1.
Shamah-Levy T, Gaona-Pineda EB, Rodríguez-Ramírez S, Morales-Ruan C, Cuevas-Nasu L, Méndez-Gómez-Humarán I, et al. Sobrepeso, obesidad y consumo de azúcares en población escolar y adolescente de México. Ensanut 2020-2022 salud pública de méxico. 2023;65:570–80.
Xiao Y, Wang H, Han L, Lyu G, Li S. Effect of uric acid on lipid metabolism assessed via restricted cubic splines: A new insight. Heliyon. 2024;10:e37408.
Saito Y, Noguchi N, Niki E. Cholesterol is more readily oxidized than phospholipid linoleates in cell membranes to produce cholesterol hydroperoxides. Free Radic Biol Med. 2024;211:89–95.
Patterson RA, Horsley ET, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: important role of uric acid. J Lipid Res. 2003;44:512–21.
Kellogg EW 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977;252:6721–8.
Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA 1988;85:9748–52.
Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–54.
Acknowledgements
We thank all the children who participated in this study and their parents or guardians for their valuable support and commitment. We would like to thank MD. Andrés Rocha Aguado, the nutritionist Evelyn Lizeth Martínez Cisneros, M.D. Brenda Valdez Feregrino (Family Medicine) and the staff of the clinical laboratory service of UMF No. 23 (IMSS), Daniela Orozco-Colín (Universidad Autónoma Metropolitana), Monserrat Hernández Reyes (Universidad Nacional Autónoma de México), and the Federal Educational Authorities of Mexico City for their invaluable efforts and contributions to this work.
Funding
This work was supported by grants from the Instituto Mexicano del Seguro Social (IMSS) under the program Temas Prioritarios en Salud 2018 (Grant No. FIS/IMSS/PROT/PRIO/18/079). A.N.C. (Ciencias Médicas Odontológicas y de la Salud PhD program from Universidad Nacional Autónoma de México) was supported by PhD fellowships from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI). A.R.C. was supported by Investigadoras e Investigadores por México fellowships from the SECIHTI.
Author information
Authors and Affiliations
Contributions
A.N.C., M.V.M. and M.C. designed the study, performed the statistical analysis, wrote the manuscript, and designed tables and figures. A.N.C., M.V.M., A.R.C., A.P.B., A.C.A., G.C.D. and E.F.G. collected the data, performed the experiments, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research protocol was approved by the National Scientific Research Commission and the Ethics Commission of the IMSS (CONBIOETICA-09-CEI-009-20160601; Registration number R-2016-785-100) and was conducted in compliance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nava-Cabrera, A., Ramírez-Cruz, A., Pérez-Bautista, A. et al. Association of serum total antioxidant capacity and uric acid with obesity and cardiometabolic traits in Mexican children. Eur J Clin Nutr 80, 73–78 (2026). https://doi.org/10.1038/s41430-025-01651-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-025-01651-9