Abstract
Megacystis–microcolon–intestinal hypoperistalsis syndrome (MMIHS) is a severe disease characterized by functional obstruction in the urinary and gastrointestinal tract. The molecular basis of this condition started to be defined recently, and the genes related to the syndrome (ACTG2—heterozygous variant in sporadic cases; and MYH11 (myosin heavy chain 11), LMOD1 (leiomodin 1) and MYLK (myosin light chain (MLC) kinase)—autosomal recessive inheritance), encode proteins involved in the smooth muscle contraction, supporting a myopathic basis for the disease. In the present article, we described a family with two affected siblings with MMIHS born to consanguineous parents and the molecular investigation performed to define the genetic etiology. Previous whole exome sequencing of the affected child and parents did not identify a candidate gene for the disease in this family, but now we present a reanalysis of the data that led to the identification of a homozygous deletion encompassing the last exon of MYL9 (myosin regulatory light chain 9) in the affected individual. MYL9 gene encodes a regulatory myosin MLC and the phosphorylation of this protein is a crucial step in the contraction process of smooth muscle cell. Despite the absence of human or animal phenotype related to MYL9, a cause–effect relationship between MYL9 and the MMIHS seems biologically plausible. The present study reveals a strong candidate gene for autosomal recessive forms of MMIHS, expanding the molecular basis of this disease and reinforces the myopathic basis of this condition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Berdon WE, Baker DH, Blanc WA, Gay B, Santulli TV, Donovan C. Megacystis–microcolon–intestinal hypoperistalsis syndrome: a new cause of intestinal obstruction in the newborn. Report of radiologic findings in five newborn girls. Am J Roentgenol. 1976;126:957–64.
Gosemann JH, Puri P. Megacystis–microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int. 2011;27:1041–6.
Moreno CA, Metze K, Lomazi EA, Bertola DR, Barbosa RH, Cosentino V, et al. Visceral myopathy: clinical and molecular survey of a cohort of seven new patients and state of the art of overlapping phenotypes. Am J Med Genet A. 2016;170:2965–74.
Thorson W, Diaz O, Joseph H, Ii F, Spiliopoulos M, Quintero R, et al. De novo ACTG2 mutations cause congenital distended bladder, microcolon, and intestinal hypoperistalsis. Hum Genet.. 2014;133:737–42.
Wangler MF, Gonzaga-Jauregui C, Gambin T, Penney S, Moss T, Chopra A, et al. Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis–microcolon–intestinal hypoperistalsis syndrome. PLoS Genet.. 2014;10:e1004258.
Gauthier J, Ouled AB, Hamdan FF, Harrison SM, Baker LA, Couture F, et al. A homozygous loss-of-function variant in MYH11 in a case with megacystis–microcolon–intestinal hypoperistalsis syndrome. Eur J Hum Genet. 2015;23:1266–8.
Halim D, Wilson MP, Oliver D, Brosens E, Verheij JB, Han Y, et al. Loss of LMOD1 impairs smooth muscle cytocontractility and causes megacystis-microcolon intestinal hypoperistalsis syndrome in humans and mice. Proc Natl Acad Sci USA. 2017;114:e2739–47.
Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, et al. Loss-of-function variants in MYLK cause recessive megacystis microcolon intestinal hypoperistalsis syndrome. Am J Hum Genet. 2017;101:123–9.
Mc Laughlin D, Puri P. Familial megacystis microcolon intestinal hypoperistalsis syndrome: a systematic review. Pedia Surg Int. 2013;29:947–51.
Halim D, Hofstra RM, Signorile L, Verdijk RM, van der Werf CS, Sribudiani Y, et al. ACTG2 variants impair actin polymerization in sporadic megacystis microcolon intestinal hypoperistalsis syndrome. Hum Mol Genet. 2016;25:571–83.
Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–45.
Ikebe M, Hartshorne DJ, Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-dalton light chain of smooth muscle myosin. J Biol Chem. 1986;261:36–39.
Kumar CC, Mohan SR, Zavodny PJ, Narula SK, Leibowitz PJ. Characterization and differential expression of human vascular smooth muscle myosin light chain 2 isoform in nonmuscle cells. Biochemistry.. 1989;28:4027–35.
Narayanan M, Murphy MS, Ainsworth JR, Arul GS. Mydriasis in association with MMIHS in a female infant: evidence for involvement of the neuronal nicotinic acetylcholine receptor. J Pediatr Surg. 2007;42:1288–90.
McClelland C, Walsh RD, Chikwava KR, Johnson MP, Mattei P, Liu GT. Congenital mydriasis associated with megacystis microcolon intestinal hypoperistalsis syndrome. J Neuroophthal. 2013;33:271–5.
Tuzovic L, Tang S, Miller RS, Rohena L, Shahmirzadi L, Gonzalez K, et al. New insights into the genetics of fetal megacystis: ACTG2 mutations, encoding γ-2 smooth muscle actin in megacystis microcolon intestinal hypoperistalsis syndrome (Berdon Syndrome). Fetal Diagn Ther. 2015;38:296–306.
Matera I, Rusmini M, Guo Y, Lerone M, Li J, Zhang J, et al. Variants of the ACTG2 gene correlate with degree of severity and presence of megacystis in chronic intestinal pseudo-obstruction. Eur J Hum Genet. 2016;24:1211–5.
Lu W, Xiao Y, Huang J, Tao Y, Yan W, Lu L, et al. Mutation in actin γ-2 responsible for megacystis microcolon intestinal hypoperistalsis syndrome in 4 Chinese patients. J Pediatr Gastroenterol Nutr. 2016;63:624–6.
Milewicz DM, Østergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010;152A:2437–43.
Vanderkerckhove J, Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation.. 1979;14:123–33.
McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–73.
Gabbiani G, Schmid E, Winter S, Chaponnier C, de Ckhastonay C, Vandekerckhove J, et al. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci USA. 1981;78:298–302.
Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet.. 2006;38:343–9.
Wang L, Guo DC, Cao J, Gong L, Kamm KE, Regalado E, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet. 2010;87:701–7.
Morano I, Chai GX, Baltas LG, Lamounier-Zepter V, Lutsch G, Kott M, et al. Smooth muscle contraction without smooth muscle myosin. Nat Cell Biol. 2000;2:371–5.
Licht AH, Nübel T, Feldner A, Jurisch-Yaksi N, Marcello M, Demicheva E, et al. Junb regulates art erial contraction capacity, cellular contractility, and motility via its target Myl9 in mice. J Clin Invest. 2010;120:2307–18.
Chen L, Fan Y, Wan J. Screening of key genes of unruptured intracranial aneurysms by using DNA microarray data analysis techniques. Genet Mol Res. 2014;13:758–67.
Marston SB, Redwood CS. The molecular anatomy of caldesmon. Biochem J.. 1991;279:1–16.
Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201–6.
Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci.. 2010;154:3–13.
Acknowledgements
The authors thank the family for their participation in this study, Prof. Íscia Lopes-Cendes for contributing to the CMA, Prof. Robert Pogue for reviewing the manuscript, and Mr. Mario Moreira da Silva for helping to make the Fig. 3. The grant sponsors of this work were: National Human Genome Research Institute—NHGRI (1U54HG006542); Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (590148/2011-7); Fundo de Apoio ao Ensino, à Pesquisa e Extensão—FAEPEX (0348/16).
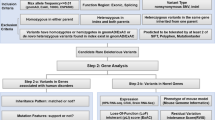
Contraction of smooth muscle cell: cellular pathway and the role of MYL9 and of other genes related to MMIHS. a Diagram illustrating some steps in the cellular pathway related to smooth muscle contraction. The phosphorylation of regulatory myosin light chain (rMLC) encoded by MYL9 is crucial to promote contraction and occurs at the end of this pathway (thick arrow). The increase in intracellular calcium concentration [Ca2+]i is an essential step to initiate the process, which is triggered by several factors (autonomic nervous system, hormones, local chemical, or mechanical stimulus). The [Ca2+]i increases due to two mechanisms: the influx of calcium ions (Ca2+) from extracellular to cytosol mediated by different channels (Ca2+ voltage-dependent and nonselective cation channels) and by the release of this ion from sarcoplasmatic reticulum (SR) through a G-protein- (G) mediated cascade. The binding of agonists to the G-protein-coupled receptor increases the phospholipase C (PLC) activity via G-protein leading to the production of inositol 1,4,5-triphophate (IP3) and diacylglycerol (DAG) from membrane phospholipids—phosphatidylinositol 4,5-biphosphate (PIP2). Protein G also activates the Rho pathway. IP3 binds to a specific receptor (IP3R) on SR causing the release of Ca2+ to the cytosol. The activation of the ryanodine receptor (RyR) on SR also contributes to the release of Ca2+ to the cytosol and both, IPR3 and RyR, are activated by increased [Ca2+]i (not shown). The intracellular Ca2+ (four molecules) binds to calmodulin (CaM) and this complex activates the myosin light chain kinase (MLCK). Activated MLCK phosphorylates the rMLC of myosin class II (also composed by myosin heavy chain—MHC and essential myosin light chain—eMLC), allowing actin–myosin binding and generating the force necessary to contract the cell. Dephosphorylation of rMLC occurs by the action of the myosin light chain phosphatase (MLCP), inducing the relaxation. MLCP is inactivated via phosphorylation mediated by kinases (PKC, RhoK, and ZIPK—activated by DAG and Rho G pathway) and activated by the cyclic nucleotide-dependent pathway. The increase in [Ca2+]i also stimulates the contraction by the pathway related to caldesmon, an actin-binding protein (not shown). b The presumed effect in smooth muscle contraction due to deletion in MYL9: the disruption of rMLC caused by deletion impairs the phosphorylation mediated by MLCK, leading to reduced or absent contraction. c The genes and the respective contractile filaments and inheritance patterns related to MMIHS—ACTG2 filamentous actin (F-actin), gamma-2 isoform, MYH11 myosin heavy chain (MHC) 11, MYLK myosin light chain kinase (MLCK), MYL9 regulatory myosin light chain (rMLC), LMOD1 leiomodin 1, an actin-binding protein. *eMLC encoded by MYL6 gene, not related to MMIHS until now, AD autosomal dominant inheritance, AR autosomal recessive inheritance. The information regarding smooth muscle contraction was based on different references [11,11,, 28,29,30]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moreno, C.A., Sobreira, N., Pugh, E. et al. Homozygous deletion in MYL9 expands the molecular basis of megacystis–microcolon–intestinal hypoperistalsis syndrome. Eur J Hum Genet 26, 669–675 (2018). https://doi.org/10.1038/s41431-017-0055-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41431-017-0055-5
This article is cited by
-
cTAGE5 is essential for adipogenesis and adipose tissue development
Nature Communications (2025)
-
HEARTSVG: a fast and accurate method for identifying spatially variable genes in large-scale spatial transcriptomics
Nature Communications (2024)
-
Genome-wide analysis identifies MYH11 compound heterozygous variants leading to visceral myopathy corresponding to late-onset form of megacystis-microcolon-intestinal hypoperistalsis syndrome
Molecular Genetics and Genomics (2024)
-
Exploring the complexities of megacystis-microcolon-intestinal hypoperistalsis syndrome: insights from genetic studies
Clinical Journal of Gastroenterology (2024)
-
Multi-disciplinary Insights from the First European Forum on Visceral Myopathy 2022 Meeting
Digestive Diseases and Sciences (2023)