Abstract
Aims
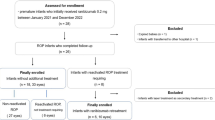
To evaluate retreatment rates, visual and anatomical outcomes at 1-year postnatal age in infants treated for retinopathy of prematurity (ROP)
Methods
Longitudinal national surveillance study of infants treated for ROP in the United Kingdom between December 2013 and December 2014, supported by the British Ophthalmic Surveillance Unit. Here we report retreatment rates, anatomical, visual and refractive outcomes at 1-year follow-up.
Results
One-year follow-up forms were completed for 168 children of the original cohort of 327 (51.4%). Twenty-two had at least one retreatment: 17/153 right eyes (REs, 11.1%) after initial diode laser, and 5/14 REs (35.7%) after initial injection of anti-vascular endothelial growth factor (VEGF) antibody. Median (interquartile range) RE best-corrected visual acuity was 0.6 (0.4–1.0) (n = 46 REs), and median acuity both eyes open 0.4 (0.3–0.7) logMAR (n = 89). Median spherical equivalent (RE) was 0.44 (−1.3 to 1.3) dioptre (D) (n = 116). Median astigmatism (RE) was 0.5 (0–1.0) D (n = 111), and median anisometropia 0.125 (0–0.75) D (n = 116). Twenty-four children (20.5%) had been prescribed glasses. Sight impairment certification eligibility information was available for 131 children: 11 (8.4%) were eligible to be certified as sight impaired, and 5 (3.8%) as severely sight impaired.
Conclusions
Retreatment rates are in line with previous reports, and appear higher after initial anti-VEGF antibody than after initial diode laser. Refractive outcomes are in line with previous studies, with a trend towards early emmetropia and myopia following diode laser, particularly in more severe ROP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Davitt BV, Dobson V, Quinn GE, Hardy RJ, Tung B, Good WV, et al. Astigmatism in the Early treatment for retinopathy of prematurity study: findings to 3 years of age. Ophthalmology. 2009;116:332–9.
Holmstrom M, el Azazi M, Kugelberg U. Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol. 1998;82:1265–71.
Saunders KJ, McCulloch DL, Shepherd AJ, Wilkinson AG. Emmetropisation following preterm birth. Br J Ophthalmol. 2002;86:1035–40.
Deng L, Gwiazda JE. Anisometropia in children from infancy to 15 years. Invest Ophthalmol Vis Sci. 2012;53:3782–7.
Mintz-Hittner HA, Kennedy KA, Chuang AZ. Group B-RC Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Eng J Med. 2011;364:603–15.
Chuluunbat T, Chan RV, Wang NK, Lien R, Chen YP, Chao AN, et al. Nonresponse and recurrence of retinopathy of prematurity after intravitreal ranibizumab treatment. Ophthalmic Surg Lasers Imaging Retina. 2016;47:1095–105.
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1327–33.
Mintz-Hittner HA, Geloneck MM, Chuang AZ. Clinical managemen of recurrent retinopathy of prematurity after intravitreal bevacizumab monotherapy. Ophthalmology. 2016;123:1845–55.
Yoon JM, Shin DH, Kim SJ, Ham DI, Kang SW, Chang YS, et al. Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina. 2017;37:88–96.
Gunay M, Sukgen EA, Celik G, Kocluk Y. Comparison of bevacizumab, ranibizumab, and laser photocoagulation in the treatment of retinopathy of prematurity in Turkey. Curr Eye Res. 2016;42:1–8.
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015;122:1008–15.
Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal low-dosage bevacizumab for retinopathy of prematurity. Acta Ophthalmol (Copenh). 2014;92:577–81.
O’Keeffe N, Murphy J, O’Keefe M, Lanigan B. Bevacizumab compared with diode laser in stage 3 posterior retinopathy of prematurity: a 5 year follow up. Ir Med J. 2016;109:355.
Sankar MJ, Sankar J, Mehta M, Bhat V, Srinivasan R. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2016;2:CD009734.
Gunay M, Celik G, Gunay BO, Aktas A, Karatekin G, Ovali F. Evaluation of 2-year outcomes following intravitreal bevacizumab (IVB) for aggressive posterior retinopathy of prematurity. Arq Bras Oftalmol. 2015;78:300–4.
Kabatas EU, Kurtul BE, Altiaylik Ozer P, Kabatas N. Comparison of intravitreal bevacizumab, intravitreal ranibizumab and laser photocoagulation for treatment of type 1 retinopathy of prematurity in Turkish preterm children. Curr Eye Res. 2017;42: 1054-1058.
Gunay M, Sekeroglu MA, Bardak H, Celik G, Esenulku CM, Hekimoglu E, et al. Evaluation of refractive errors and ocular biometric outcomes after intravitreal bevacizumab for retinopathy of prematurity. Strabismus. 2016;24:84–88.
Holmstrom G, Hellstrom A, Jakobsson P, Lundgren P, Tornqvist K, Wallin A. Five years of treatment for retinopathy of prematurity in Sweden: results from SWEDROP, a national quality register. Br J Ophthalmol. 2016;100:1656–61.
Adams GG, Bunce C, Xing W, Butler L, Long V, Reddy A, et al. Treatment trends for retinopathy of prematurity in the UK: active surveillance study of infants at risk. BMJ Open. 2017;7:e013366.
Adoh TO, Woodhouse JM. The Cardiff acuity test used for measuring visual acuity development in toddlers. Vision Res. 1994;34:555–60.
Courage ML, Adams RJ. Visual acuity assessment from birth to three years using the acuity card procedure: cross-sectional and longitudinal samples. Optom Vis Sci. 1990;67:713–8.
Sharma P, Bairagi D, Sachdeva MM, Kaur K, Khokhar S, Saxena R. Comparative evaluation of Teller and Cardiff acuity tests in normals and unilateral amblyopes in under-two-year-olds. Indian J Ophthalmol. 2003;51:341–5.
Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM, et al. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology. 2005;112:1564–8.
Quinn GE, Dobson V, Davitt BV, Hardy RJ, Tung B, Pedroza C, et al. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings to 3 years of age. Ophthalmology. 2008;115:1058–64 e1051.
Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961.
Acknowledgements
We thank the British Ophthalmic Surveillance Unit, in particular Mr Barny Foot, for advice on study design and collection of case notifications. We also thank Miss Anneka Tailor for liaising with practitioners across the UK to facilitate data collection and completion of reports, and for maintaining the study database. We thank Mr Zabed Ahmed for setting up a comprehensive electronic database, and the Moorfields Special Trustees (grant ST 14 01 D) and the Birmingham Eye Foundation for their generous funding for this work. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The study was approved by the Research Ethics Committee North of Scotland, Aberdeen (13/NS/0059). This work was registered at clinicaltrials.gov as NCT02484989.
Author contributions
GGWA, CB, LB, AHD-N, VL and AR developed the study protocol. LB, AHD-N, GGWA and CB secured funding. All authors reviewed and discussed and interpreted the data acquired. WX carried out data analysis. AHD-N drafted the manuscript, which was then critically reviewed and modified by all authors.
Members of the UK ROP Special Interest Group:
Abbott, Joseph; Aclimandos, Wagih; Adams, Gill; Al-Khaier, Ayman; Allen, Louise; Arashvan, Kayvan; Ashworth, Jane; Barampouti, Faye; Barnes, Jonathan; Barrett, Victoria; Barry, John Sebastian; Bates, Adam; Berk, Tulin; Biswas, Susmito; Blaikie, Andrew; Brennan, Rosie; Bunting, Howard; Butcher, Jeremy; Butler, Lucilla; Chan-Ling, Tailoi; Chan, Jonathan; Child, Christopher; Choi, Jessy; Clark, David; Clifford, Luke; Dabbagh, Ahmad; Dahlmann-Noor, Annegret; Dawidek, Gervase; Dhir, Luna; Drake, Karen; Edwards, Richard; Esakowitz, Leonard; Escardo-Paton, Julia; Evans, Anthony; Fleck, Brian; Geh, Vernon; George, Nick; Gnanaray, Lawrence; Goyal, Raina; Haigh, Paul; Hancox, Joanne; Haynes, Richard; Heath, Dominic; Henderson, Robert; Hillier, Roxane; Hingorani, Melanie; Jain, Saurabh; Jain, Sunila; Jones, David; Kafil-Hussain, Namir; Kelly, Simon; Kenawy, Nihal; Kipioti, Tina; Kulkarni, Archana; Lavy, Tim; Laws, David; Lawson, Joanna; Leitch, Jane; Ling, Roland; Long, Vernon; Macrae, Mary; Mahmood, Usman; Markham, Richard; Marr, Jane; May, Kristina; McLoone, Eibhlin; Moosa, Murad; Morton, Claire; Mount, Ali; Muen, Wisam; Mulvihill, Alan; Munshi, Vineeta; Muqit, Mahi; Murray, Robert; Nair, Ranjit; Newman, William; O’Colmain, Una; Patel, Chetan; Patel, Himanshu; Pedraza, Luis Amaya; Pilling, Rachel; Puvanachandra, Narman;Quinn, Anthony; Rathod, Dinesh; Reddy, Aravind; Reddy, Ashwin; Rowlands, Alison; Scotcher, Stephen; Scott, Christopher; Sekhri, Rajnish; Shafiq, Ayad; Sleep, Tamsin; Tambe, Katya; Tandon, Anamika; Tappin, Alison; Taylor, Robert; Theodoro, Maria; Thomas, Shery; Thompson, Graham; Tiffin, Peter; Ullah, Muhammed Aman; Watts, Patrick; West, Stephanie; Whyte, Iain; Wickham, Louisa; Williams, Cathy; Wong, Chien; Wren, Siobhan; Zakir, Rahila.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
This work was funded by Moorfields Eye Charity and the Birmingham Eye Foundation. There have not been any financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. The funders have not in any way influenced the study design or conduct, and the researchers are independent from the funders. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The lead author, AHDN, affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Additional information
Members of the UK Retinopathy of Prematurity Special Interest Group are listed before the references.
Electronic supplementary material
41433_2018_151_MOESM1_ESM.docx
Tables 5-6, Retreatment rates (table 5), retreatment with secondary, tertiary and any further treatment (table 6) following initial laser or anti-VEGF antibody treatment, based on 327 children of orig
Rights and permissions
About this article
Cite this article
Adams, G.G., Bunce, C., Xing, W. et al. Retinopathy of prematurity in the United Kingdom: retreatment rates, visual and structural 1-year outcomes. Eye 32, 1752–1759 (2018). https://doi.org/10.1038/s41433-018-0151-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0151-y