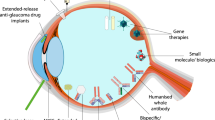
Abstract
Recent advances in pharmacological agents have led to successful treatment of a variety of retinal diseases such as neovascular age-related macular degeneration (AMD), diabetic macular oedema (DMO), and retinal vascular occlusions (RVO). These treatments often require repeated drug injections for an extended period of time. To reduce these repeated treatment burdens, minimally invasive drug delivery systems are needed. An ideal therapy should maintain effective levels of drug for the intended duration of treatment following a single application, recognising that a significant number of months of therapy may be required. There are numerous approaches under investigation to improve treatment options. This review will highlight the advantages and limitations of selected drug delivery systems of novel biomaterial implants and depots. The main emphasis will be placed on less invasive, longer acting, sustained release formulations for the treatment of retinal disorders.
摘要
近年来, 药理学方面的进步改善了多种视网膜疾病的预后, 例如新生血管性年龄相关性黄斑变性 (AMD), 糖尿病性黄斑水肿 (DMO) 和视网膜血管阻塞 (RVO)。此类疾病的治疗通常需要长期反复注射给药, 为了减少重复性治疗的负担, 尽可能使用无创的药物传输系统。考虑到可能需要数月的治疗周期, 理想的治疗方式是在单次给药后较长时间内能保持有效的药物浓度。目前多种药物正在研发之中以改善药物治疗方案。本篇综述概括了几种特定的新型生物材料植入物和缓释库给药途径的优势和局限性。重点介绍了治疗视网膜疾病的侵入性小, 作用时间长, 持续性释放的几种制剂。
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21:S3–9.
Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol 2012;763:70–84.
Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2004;4:371–88.
Yasukawa T, Ogura Y, Tabata Y, Kimuar H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res. 2004;23:253–81.
Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014;11:1647–60.
Kang-Mieler JJ, Kiernan DF, Mieler WF. Duane’s ophthalmology: drug delivery to the posterior segment. Philadelphia: Lippincott Williams & Wilkins; 2011.
Yang C, Tirucherai GS, Mitra AK. Prodrug based optial drug delivery via membrane transporter/receptor. Expert Opin Biol Ther. 2001;1:159–75.
Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114.
Kim S, Lutz R, Wang N, Robinson M. Transport barriers in transscleral drug delivery for retinal diseases. Ophthal Res. 2007;39:244–54.
Yeh S. American Society of Retina Specialists 2018 Late-breaking Presentation. Suprachoroidally injected CLS-TA improves visual acuity and macular edema in noninfectious uveitis: results of the Phase 3 PEACHTREE study. 2018.
National Library of Medicine (US). Identifier NCT02595398, suprachoroidal injection of CLS-TA in subjects with macular edema associated with non-infectious uveitis (PEACHTREE). ClinicalTrials.gov [Internet], Bethesda, MD. 2015. https://clinicaltrials.gov/ct2/show/NCT02595398?term=PEACHTREE&draw=2&rank=1. Accessed 5 Nov 2019.
Ip MS, Nittala MG, Velaga S, Ciulla T, Sadda SR, on behalf of the TYBEE Study Group American Academy of Ophthalmology 2019 Presentation PO485. Suprachoroidal CLS-TA plus aflibercept compared with aflibercept monotherapy for DME: selected secondary results of the randomized phase 2 TYBEE trial. 2019.
REGENXBIO, Rockville, MD. https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-exclusive-worldwide-option-and-license. Accessed 14 Nov 2019.
Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Two-year interim clinical study results. J Cataract Refract Surg 2009;35:814–24.
Kang SJ, Patel SR, Berezovsky DE, Zhang Q, Yang H, Grossniklaus HE. Suprachoroidal injection of microspheres with microcatheter in a rabbit model of uveal melanoma. Presented at the Association for Research in Vision and Ophthalmology Annual Meeting. Fort Lauderdale, FL: Investigative Ophthalmology & Visual Science; 2011.
Olsen TW, Feng X, Wabner K, Conston SR, Sierra DH, Folden DV, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol 2006;142:777–87.
Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci 2011;52:4749–56.
Rizzo S, Ebert FG, Bartolo ED, Barca F, Cresti F, Augustin C, et al. Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates. Retina 2012;32:776–84.
National Library of Medicine (US). Identifier NCT01226628, a study of the safety and efficacy of CNTO2476 in patients with age-related macular degeneration. ClinicalTrials.gov [Internet], 2010 October 22. Bethesda, MD. http://clinicaltrials.gov/ct2/show/NCT01226628?term=CNTO2476&rank=1. Accessed 14 Nov 2019.
Ho AC. Human adult umbilical stem cells: potential treatment for atrophic AMD. Retin Today 2011;7:59–61.
El Rayes EN. Into the Suprachoridal space. Retin Today 2018;13:28–32.
El Rayes EN. Suprachoroidal steroid delivery: enforcing our management for refractory macular edema. Retin Today 2013;8:49–51.
Grzybowski A, Told R, Sacu S, Bandello F, Moisseiev E. Loewenstein A on behalf of the Euretina Board. 2018 update on intravitreal injections: euretina expert consensus recommendations. Ophthalmologica. 2018;239:181–93.
Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol 2008;86:372–6.
Bourges J, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–202.
Lim JI, Wolitz RA, Dowling AH, Bloom HR, Irvine AR, Schwartz DM. Visual and anatomic outcomes associated with posterior segment complications after ganciclovir implant procedures in patients with AIDS and cytomegalovirus retinitis. Am J Ophthalmol. 1999;127:288–93.
Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. Ocular pharmacokinetics of fluocinolone acetonide after RetisertTM intravitreal implantation in rabbits over a 1-year period. J Ocul Pharm Ther. 2004;20:269–75.
Ebrahim S, Peyman GA, Lee PJ. Applications of liposomes in ophthalmology. Surv Ophthalmol. 2005;50:167–82.
Emerich DF, Thanos C. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther. 2008;10:506–15.
Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–26.
Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–901.
Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, Stabilia P, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative disease. Invest Ophthalmol Vis Sci 2012;53:7484–91.
National Library of Medicine (US). Identifier NCT02862938, study of NT-501 encapsulated cell therapy for glaucoma neuroprotection and vision restoration. ClinicalTrials.gov [Internet]. Bethesda, MD. 2016. https://clinicaltrials.gov/ct2/show/NCT02862938?cond=NT-501&rank=2. Accessed 4 Nov 2019.
National Library of Medicine (US). Identifier NCT03071965, Extension study of NT-501 ciliary neurotrophic factor (CNTF) implant for macular telangiectasia (MacTel). ClinicalTrials.gov [Internet]. Bethesda, MD. 2017. https://clinicaltrials.gov/ct2/show/NCT03071965?cond=NT-501&rank=1. Accessed 4 Nov 2019.
Chew EY, Clemons TE, Jaffe GJ, Johnson CA, Farsiu S, Lad EM, on behalf of the Macular Telangiectasia Type 2-Phase 2 CNTF Research Group. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology. 2019;126:540–9.
Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol 2008;126:1191–201.
Haghjou N, Soheilian M, Abdekhodaie MJ. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res 2011;6:317–29.
Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112:1192–98.
Retisert (fluocinolone acetonide intravitreal implant) 0.59 mg, Bausch & Lomb, Inc., Rochester, NY. 2014. https://www.bausch.com/ecp/our-products/rx-pharmaceuticals/rx-pharmaceuticals/retisert-fluocinolone-acetonide-intravitreal-implant-059-mg#.Xc2tIHv_pPY. Accessed 14 Nov 2019.
Kane FE, Burdan J, Cutino A, Green KE. Iluvien™: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv. 2008;5:1039–46.
Kuppermann BD, Blumenkranz MS, Haller JA, Williams GA, Weinberg DV, Chou C, on behalf of the Dexamethasone DDS Phase II Study Group. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125:309–17.
Bucolo C, Drago F, Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012;3:188.
Mi FL, Lin YM, Wu YB, Shyu SS, Tsai YH. Chitin/PLGA blend microspheres as a biodegradable drug-delivery system: phase-separation, degradation and release behavior. Biomaterials. 2002;23:3257–67.
Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–27.
Herrero-Vanrell R, Bravo-Osuna I, Andres-Guerrero V, Vicario-de-la-Torre M, Molina-Martinez IT. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog Retin Eye Res 2014;42:27–43.
Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res 2004;27:1–12.
Manoharan C, Singh J. Insulin loaded PLGA microspheres: effect of zinc salts on encapsulation, release, and stability. J Pharm Sci 2009;98:529–42.
Varshochian R, Jeddi-Tehrani M, Mahmoudi AR, Khoshayand MR, Atyabi F, Sabzevari A, et al. The protective effect of albumin on bevacizumab activity and stability in PLGA nanoparticles intended for retinal and choroidal neovascularization treatments. Eur J Pharm Sci 2013;50:341–52.
Varshochian R, Riazi-Esfahani M, Jeddi-Tehrani M, Mahmoudi AR, Aghazadeh S, Mahbod M, et al. Albuminated PLGA nanoparticles containing bevacizumab intended for ocular neovascularization treatment. J Biomed Mater Res A 2015;103:3148–56.
Ye Z, Ji Y-L, Ma X, Wen J-G, Wei W, Huang S-M. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int J Ophthalmol 2015;8:653–8.
GB-102, Garybug Vision, Redwood City, CA. https://graybug.com. Accessed 14 Nov 2019.
National Library of Medicine (US). Identifier NCT03249740, a depot formulation of sunitinib malate (GB-102) in subjects with neovascular (wet) age-related macular degeneration. ClinicalTrials.gov [Internet], Bethesda, MD. 2017. https://clinicaltrials.gov/ct2/show/NCT03249740?term=graybug+vision&cntry=US&draw=2&rank=2. Accessed 14 Nov 2019.
National Library of Medicine (US). Identifier NCT03953079, a depot formulation of sunitinib malate (GB-102) compared to aflibercept in subjects with wet AMD (ALTISSIMO). ClinicalTrials.gov [Internet]. Bethesda, MD. 2019. https://clinicaltrials.gov/ct2/show/NCT03953079. Accessed 14 Nov 2019.
OTX-IVT (anti-VEGF antibody implant), Ocular Therapeutix, Bedford, MA. https://ocutx.com/research/otx-ivt/. Accessed 7 Nov 2019.
El-Hayek R, Jarrett T, Lattrell Z, Takach S, Jarret PK, McGrath M, et al. Efficacy of a 6 month sustained hydrogel delivery system for Tyrosine kinase inhibitors in a VEGF induced retinal leakage model. ARVO Abstract. Invest Ophthalmol Vis Sci 2017;58:1968.
Ocular Therapeutix announces dosing of first patient in Phase 1 clinical trial for the treatment of wet AMD. Eyewire News. https://eyewire.news/articles/ocular-therapeutix-announces-dosing-of-first-patient-in-phase-1-clinical-trial-for-the-treatment-of-wet-amd/. Accessed 2 Feb 2019.
Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv 2012;9:383–402.
Klouda L. Thermoresponsive hydrogels in biomedical applications a seven-year update. Eur J Pharm Biopharm. 2015;97:338–49.
Drapala PW, Brey EM, Mieler WF, Venerus DC, Kang-Derwent JJ, Perez-Luna VH. Role of thermo-responsiveness and poly(ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J Biomater Sci Polym Ed 2011;22:59–75.
Drapala PW, Jiang B, Chiu YC, Mieler WF, Brey EM, Kang-Mieler JJ, et al. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm Res 2014;31:742–53.
Turturro SB, Guthrie MJ, Appel AA, Drapala PW, Brey EM, Perez-Luna VH, et al. The effect of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 2011;32:3620–6.
Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules 2012;13:40–48.
Yu Y, Lau LC, Lo AC, Chau Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6-month in vivo study. Transl Vis Sci Technol 2015;4:5.
Moritera T, Ogura Y, Hondo Y, Wadaf R, Hyoaf S-H, Ikadaf Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest Ophthalmol Vis Sci 1991;32:1785–90.
Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF. Extended ocular drug delivery systems for the anterior and posterior segments: biomaterial options and applications. Expert Opin Drug Deliv 2017;14:611–20.
Osswald CR, Kang-Mieler JJ. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. Ann Biomed Eng 2015;43:2609–17.
Osswald CR, Kang-Mieler JJ. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res 2016;41:1216–22.
Liu W, Borrell MA, Venerus DC, Mieler WF, Kang-Mieler JJ. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl Vis Sci Technol 2019;8:12.
Osswald CR, Guthrie MJ, Avila A, Valio JA Jr, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res 2017;42:1293–301.
Liu W, Lee BS, Mieler WF, Kang-Mieler WF. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr Eye Res 2019;44:264–74.
Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized Phase 2 Ladder clinical trial. Ophthalmology. 2019;126:1141–54.
Rubio RG. Long-acting anti-VEGF delivery. Retin Today. 2014;8:78–80.
Funding
We would like to acknowledge NIH Research Grants (EY025434 and EY029298). Jennifer J. Kang-Mieler: NIH Research Grants (EY025434; EY029298). Kayla M. Rudeen, Wenqiang Liu, William F. Mieler: None
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jennifer J. Kang-Mieler: US Patents pending on “Microsphere-hydrogel drug delivery system”. Kayla M. Rudeen, Wenqiang Liu, William F. Mieler: None.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang-Mieler, J.J., Rudeen, K.M., Liu, W. et al. Advances in ocular drug delivery systems. Eye 34, 1371–1379 (2020). https://doi.org/10.1038/s41433-020-0809-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-020-0809-0
This article is cited by
-
Innovative triamcinolone acetonide microsuspension for Non-Invasive ocular management of inflammation
DARU Journal of Pharmaceutical Sciences (2025)
-
Undaria pinnatifida fucoidan extract inhibits activation of the NF-κB signaling pathway by herpes simplex virus type 1 and prevents amyloid-β peptide synthesis in retinal pigment epithelium cells
Archives of Virology (2025)
-
Recent updates on drug delivery approaches for improved ocular delivery with an insight into nanostructured drug delivery carriers for anterior and posterior segment disorders
Drug Delivery and Translational Research (2025)
-
Nanotechnology for vision restoration
Nature Reviews Bioengineering (2024)
-
A wireless battery-free eye modulation patch for high myopia therapy
Nature Communications (2024)