Abstract
Objectives
To observe the changes of viral load in aqueous humour samples and visual outcomes in varicella zoster virus (VZV)-induced acute retinal necrosis (ARN).
Methods
Observational retrospective study. Medical records and viral load measured by real-time quantitative polymerase chain reaction (qPCR) of 20 eyes with VZV-induced ARN were reviewed.
Results
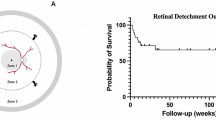
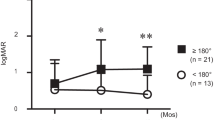
The mean viral load at presentation was 5.7 × 107 ± 9.7 × 107 copies/mL. An initial plateau phase for viral load lasting up to 2 weeks occurred in most eyes (18 eyes, 90%). In the following logarithmic reduction phase, the mean slope of the decline in viral load was −0.103 ± 0.029 log/day, and the expected time for half reduction of the initial viral load was 3.2 ± 1.0 days. At the end of the first 8-week’s antiviral treatment, the viral load was below detection threshold in all 20 eyes (100.0%). The mean logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) improved from 1.1 ± 0.7 (Snellen equivalent 20/250) to 0.7 ± 0.6 (Snellen equivalent 20/100) after a follow-up of 8.6 ± 2.0 months. Thirteen of the 20 eyes (65.0%) suffered retinal detachment and underwent vitrectomy. The initial viral load was the independent predictive factor of logMAR BCVA at the last follow-up (β = 0.745, P < 0.001).
Conclusions
The observation of viral load changes by qPCR was useful for better monitoring of therapeutic efficacy and deciding needed antiviral duration in VZV-induced ARN patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Urayama A. Unilateral acute uveitis with retinal peri-arteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607e19.
Schoenberger SD, Kim SJ, Thorne JE, Mruthyunjaya P, Yeh S, Bakri SJ, et al. Diagnosis and Treatment of Acute Retinal Necrosis: a Report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:382–92.
Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117:663–7.
Wong RW, Jumper JM, McDonald HR, Johnson RN, Fu A, Lujan BJ, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol. 2013;97:545–52.
Dworkin LL, Gibler TM, Van Gelder RN. Real-time quantitative polymerase chain reaction diagnosis of infectious posterior uveitis. Arch Ophthalmol. 2002;120:1534–9.
Asano S, Yoshikawa T, Kimura H, Enomoto Y, Ohashi M, Terasaki H, et al. Monitoring herpesvirus DNA in three cases of acute retinal necrosis by real-time PCR. J Clin Virol. 2004;29:206–9.
Cottet L, Kaiser L, Hirsch HH, Baglivo E. HSV2 acute retinal necrosis: diagnosis and monitoring with quantitative polymerase chain reaction. Int Ophthalmol. 2009;29:199–201.
Holland GN, Buhles WC Jr, Mastre B, Kaplan HJ. UCLA CMV Retinopathy Study Group. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. Arch Ophthalmol. 1989;107:1759–66.
Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30:287–90.
Espy MJ, Teo R, Ross TK, Svien KA, Wold AD, Uhl JR, et al. Diagnosis of varicella-zoster virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38:3187–9.
Kido S, Sugita S, Horie S, Miyanaga M, Miyata K, Shimizu N, et al. Association of varicella zoster virus load in the aqueous humour with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br J Ophthalmol. 2008;92:505–8.
Roberts TC, Brennan DC, Buller RS, Gaudreault-Keener M, Schnitzler MA, Sternhell KE, et al. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J Infect Dis. 1998;178:626–35.
Bernheim D, Germi R, Labetoulle M, Romanet JP, Morand P, Chiquet C. Time profile of viral DNA in aqueous humour samples of patients treated for varicella-zoster virus acute retinal necrosis by use of quantitative real-time PCR. J Clin Microbiol. 2013;51:2160–6.
Calvo CM, Khan MA, Mehta S, Garg SJ, Dunn JP. Correlation of Clinical Outcomes with Quantitative Polymerase Chain Reaction DNA Copy Number in Patients with Acute Retinal Necrosis. Ocul Immunol Inflamm. 2017;25:246–52.
Rochat C, Polla BS, Herbort CP. Immunological profiles in patients with acute retinal necrosis. Graefes Arch Clin Exp Ophthalmol. 1996;234:547–52.
Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–65.
Hafidi M, Manificat HJ, Denis P, Charleux B, Rabilloud M, Boibieux A, et al. Acute Retinal Necrosis: virological Features Using Quantitative Polymerase Chain Reaction, Therapeutic Management, and Clinical Outcomes. Am J Ophthalmol. 2019;208:376–86.
Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of acute retinal necrosis. Ophthalmology. 2010;117:818–24.
Andrei G, Topalis D, Fiten P, McGuigan C, Balzarini J, Opdenakker G, et al. In vitro-selected drug-resistant varicella-zoster virus mutants in the thymidine kinase and DNA polymerase genes yield novel phenotype-genotype associations and highlight differences between antiherpesvirus drugs. J Virol. 2012;86:2641–52.
Piret J, Boivin G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management. Curr Opin Infect Dis. 2016;29:654–62.
Mercier-Darty M, Boutolleau D, Lepeule R, Rodriguez C, Burrel S. Utility of ultra-deep sequencing for detection of varicella-zoster virus antiviral resistance mutations. Antivir Res. 2018;151:20–23.
Mercier-Darty M, Boutolleau D, Rodriguez C, Burrel S. Added value of ultra-deep sequencing (UDS) approach for detection of genotypic antiviral resistance of herpes simplex virus (HSV). Antivir Res. 2019;168:128–33.
Roy R, Pal BP, Mathur G, Rao C, Das D, Biswas J. Acute retinal necrosis: clinical features, management and outcomes-a 10-year consecutive case series. Ocul Immunol Inflamm. 2014;22:170–4.
Baltinas J, Lightman S, Tomkins-Netzer O. Comparing Treatment of Acute Retinal Necrosis With Either Oral Valacyclovir or Intravenous Acyclovir. Am J Ophthalmol. 2018;188:173–80.
Lei BY, Jiang R, Wang ZJ, Xu GZ, Wu XY, Zhou M. Bilateral acute retinal necrosis: a case series. Retina. 2020;40:145–53.
Palay DA, Sternberg P Jr, Davis J, Lewis H, Holland GN, Mieler WF, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112:250–5.
Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114:756–62.
Hillenkamp J, Nölle B, Bruns C, Rautenberg P, Fickenscher H, Roider J. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009;116:1971–15.
Acknowledgements
The authors thank Jianhong Dong and Zhijian Jiang (Shanghai Xuhui Central Hospital) for collecting data, and thank all patients involved in the study for their contributions in sample collection.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 81770944 and 81700862), Grant from Science and Technology Commission of Shanghai Municipality (Grant no. 16411953700 and 18411965100), Xuhui District Health and Family Planning Commission Key Disease Joint Project (XHLHGG201807).
Author information
Authors and Affiliations
Contributions
BL and ZW contributed equally to the paper. BL and MZ designed the study. The main part of the paper was drafted by BL and ZW. The paper was revised by QC, GX and MZ. Clinical data were collected, interpretated and added to the paper by BL, QS, RG, YZ and RJ. PCR analysis were conducted by ZW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, B., Wang, Z., Shu, Q. et al. Observation of varicella zoster virus-induced acute retinal necrosis: viral load detection and visual outcome. Eye 36, 1209–1216 (2022). https://doi.org/10.1038/s41433-021-01609-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-021-01609-8
This article is cited by
-
Clinical characteristics and outcomes of acute retinal necrosis at different stages: a retrospective study
BMC Ophthalmology (2025)
-
Correlation between interleukins in aqueous humor and vitreous humor of vitreoretinal lymphoma patients
Eye and Vision (2025)
-
Prognostic factors associated with acute retinal necrosis treated non-surgically
Eye (2024)
-
Factors at the initial visit associated with poor visual outcomes in patients with acute retinal necrosis
Eye (2024)
-
False negative result of polymerase chain reaction in very early stages of acute retinal necrosis
Journal of Ophthalmic Inflammation and Infection (2023)