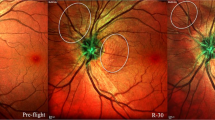
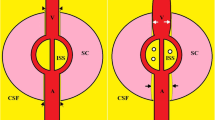
Abstract
The National Aeronautics and Space Administration (NASA) in the United States has been studying a fascinating and unique constellation of neuro-ophthalmic findings collectively known as Spaceflight Associated Neuro-Ocular Syndrome (SANS). SANS is unique to the space environment of microgravity and produces novel physiological and pathological findings that have no direct terrestrial equivalent. The neuro-ophthalmic phenomenon is a major physiologic barrier to future planetary spaceflight. The underlying pathophysiology of SANS remains ill-defined, but since its initial report in 2011, several hypotheses have been proposed including increased intracranial pressure, cerebral venous congestion and glymphatic stasis, compartmentalization of CSF within the orbital nerve sheath sub-arachnoid space (SAS), upward brain shift, inflammation, disrupted axoplasmic transport, and radiation exposure. These aetiologies may not be mutually exclusive and may be interconnected, leading to an integrative, multifactorial aetiology of SANS. This paper critically analyses the various hypotheses of this neuro-ophthalmic phenomenon and the connections between the physiologic and anatomical evidence-based changes observed in spaceflight and terrestrial analogues. Continued prospective, longitudinal study and development of practical countermeasures for SANS will be necessary for future human spaceflight missions including the mission to Mars.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Patel ZS, Brunstetter TJ, Tarver WJ, Whitmire AM, Zwart SR, Smith SM. et al. Red risks for a journey to the red planet: The highest priority human health risks for a mission to Mars. NPJ Microgravity. 2020;6:33. https://doi.org/10.1038/s41526-020-00124-6.
Law J, Mathers CH, Fondy SR, Vanderploeg JM, Kerstman EL. NASA’s human system risk management approach and its applicability to commercial spaceflight. Aviat Space Environ Med. 2013;84:68–73. https://doi.org/10.3357/asem.3421.2013.
Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J. et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118:2058–69. https://doi.org/10.1016/j.ophtha.2011.06.021.
Ong J, Tarver W, Brunstetter T, Mader TH, Gibson CR, Mason SS. et al. Spaceflight associated neuro-ocular syndrome: proposed pathogenesis, terrestrial analogues, and emerging countermeasures. Br J Ophthalmol. 2023;107:895–900. https://doi.org/10.1136/bjo-2022-322892.
Mader TH, Gibson CR, Otto CA, Sargsyan AE, Miller NR, Subramanian PS. et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol. 2017;37:133–9.https://doi.org/10.1097/WNO.0000000000000467.
Mader TH, Gibson CR, Barratt MR, Miller NR, Subramanian PS, Killer HE. et al. Persistent globe flattening in astronauts following long-duration spaceflight. Neuroophthalmology. 2021;45:29–35. https://doi.org/10.1080/01658107.2020.1791189.
Mader TH, Gibson CR, Pass AF, Lee AG, Killer HE, Hansen HC. et al. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol. 2013;33:249–55. https://doi.org/10.1097/WNO.0b013e31829b41a6.
Lee AG, Mader TH, Gibson CR, Tarver W, Rabiei P, Riascos RF. et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. NPJ Microgravity. 2020;6:7. https://doi.org/10.1038/s41526-020-0097-9.
Ong J, Mader TH, Gibson CR, Mason SS, Lee AG. Spaceflight associated neuro-ocular syndrome (SANS): an update on potential microgravity-based pathophysiology and mitigation development. Eye. 2023;37:2409–15. https://doi.org/10.1038/s41433-023-02522-y.
Lee AG, Tarver WJ, Mader TH, Gibson CR, Hart SF, Otto CA. Neuro-ophthalmology of space flight. J Neuroophthalmol. 2016;36:85–91. https://doi.org/10.1097/WNO.0000000000000334.
Ong J, Tavakkoli A, Strangman G, Zaman N, Kamran SA, Zhang Q. et al. Neuro-ophthalmic imaging and visual assessment technology for spaceflight associated neuro-ocular syndrome (SANS). Surv Ophthalmol. 2022;67:1443–66. https://doi.org/10.1016/j.survophthal.2022.04.004.
Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology. 2012;263:819–27. https://doi.org/10.1148/radiol.12111986.
Roberts DR, Albrecht MH, Collins HR, Asemani D, Chatterjee AR, Spampinato MV. et al. Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med. 2017;377:1746–53. https://doi.org/10.1056/NEJMoa1705129.
HRP, NASA. Human Research Roadmap. 2021. https://humanresearchroadmap.nasa.gov/Gaps/gap.aspx?i=518.
Iwasaki K, Levine BD, Zhang R, Zuckerman JH, Pawelczyk JA, Diedrich A. et al. Human cerebral autoregulation before, during and after spaceflight. J Physiol. 2007;579:799–810. https://doi.org/10.1113/jphysiol.2006.119636.
Nelson ES, Mulugeta L, Myers JG. Microgravity-induced fluid shift and ophthalmic changes. Life. 2014;4:621–65. https://doi.org/10.3390/life4040621.
Zhang LF, Hargens AR. Spaceflight-induced intracranial hypertension and visual impairment: pathophysiology and countermeasures. Physiol Rev. 2018;98:59–87. https://doi.org/10.1152/physrev.00017.2016.
Shinojima A, Kakeya I, Tada S. Association of space flight with problems of the brain and eyes. JAMA Ophthalmol. 2018;136:1075–6. https://doi.org/10.1001/jamaophthalmol.2018.2635.
Taniguchi-Shinojima, A Mechanical alterations of the brain and optic chiasm in Spaceflight Associated Neuro-Ocular Syndrome. In Lee, AG & Ong, J, editors. Spaceflight Associated Neuro-Ocular Syndrome. Cambridge, MA: Academic Press; 2022. p. 77–84. https://doi.org/10.1016/B978-0-323-91524-3.00014-4.
Wostyn P, Mader TH, Gibson CR, Nedergaard M. Does long-duration exposure to microgravity lead to dysregulation of the brain and ocular glymphatic systems? Eye Brain. 2022;14:49–58. https://doi.org/10.2147/EB.S354710.
Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón-Chancellor SM, Ebert DJ, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open. 2019;2:e1915011 https://doi.org/10.1001/jamanetworkopen.2019.15011.
Watkins W, Hargens AR, Seidl S, Clary EM, Macias BR. Lower-body negative pressure decreases noninvasively measured intracranial pressure and internal jugular vein cross-sectional area during head-down tilt. J Appl Physiol (1985). 2017;123:260–6. https://doi.org/10.1152/japplphysiol.00091.2017.
Harris KM, Petersen LG, Weber T. Reviving lower body negative pressure as a countermeasure to prevent pathological vascular and ocular changes in microgravity. NPJ Microgravity. 2020;6:38 https://doi.org/10.1038/s41526-020-00127-3.
Martin, DS, Lee SM, Matz TP, Westby CM, Scott JM, Stenger MB et al. Internal jugular pressure increases during parabolic flight. Physiol Rep. 2016;4. https://doi.org/10.14814/phy2.13068.
Lal SM, Twardowski ZJ, Van Stone J, Keniston D, Scott WJ, Berg GG. et al. Benign intracranial hypertension: a complication of subclavian vein catheterization and arteriovenous fistula. Am J Kidney Dis. 1986;8:262–4. https://doi.org/10.1016/s0272-6386(86)80037-3.
Cuadra SA, Padberg FT, Turbin RE, Farkas J, Frohman LP. Cerebral venous hypertension and blindness: a reversible complication. J Vasc Surg. 2005;42:792–5. https://doi.org/10.1016/j.jvs.2005.05.060.
Cleper R, Goldenberg-Cohen N, Kornreich L, Krause I, Davidovits M. Neurologic and ophthalmologic complications of vascular access in a hemodialysis patient. Pediatr Nephrol. 2007;22:1377–82. https://doi.org/10.1007/s00467-007-0491-5.
Kramer LA, Hasan KM, Stenger MB, Sargsyan A, Laurie SS, Otto C, et al. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology. 2020;295:640–8. https://doi.org/10.1148/radiol.2020191413.
Hansen HC, Lagreze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol. 2011;89:e528–532. https://doi.org/10.1111/j.1755-3768.2011.02159.x.
Hiles LA, Donoviel DB, Bershad EM. Noninvasive brain physiology monitoring for extreme environments: a critical review. J Neurosurg Anesthesiol. 2015;27:318–28. https://doi.org/10.1097/ANA.0000000000000175.
Lerner DJ, Chima RS, Patel K, Parmet AJ. Ultrasound guided lumbar puncture and remote guidance for potential in-flight evaluation of VIIP/SANS. Aerosp Med Hum Perform. 2019;90:58–62. https://doi.org/10.3357/AMHP.5170.2019.
Ong, J, Waisberg E, Masalkhi M, Kamran SA, Lowry K, Sarker P, et al. Artificial intelligence frameworks to detect and investigate the pathophysiology of spaceflight associated neuro-ocular syndrome (SANS). Brain Sci. 2023;13. https://doi.org/10.3390/brainsci13081148.
Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595:2115–27. https://doi.org/10.1113/JP273557.
Zhao, D, He, Z, Vingrys, AJ, Bui, BV & Nguyen, CT The effect of intraocular and intracranial pressure on retinal structure and function in rats. Physiol Rep. 2015;3. https://doi.org/10.14814/phy2.12507.
Berdahl JP, Yu DY, Morgan WH. The translaminar pressure gradient in sustained zero gravity, idiopathic intracranial hypertension, and glaucoma. Med Hypotheses. 2012;79:719–24. https://doi.org/10.1016/j.mehy.2012.08.009.
Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res. 2016;50:108–44. https://doi.org/10.1016/j.preteyeres.2015.10.001.
Thurtell MJ, Bruce BB, Newman NJ, Biousse V. An update on idiopathic intracranial hypertension. Rev Neurol Dis. 2010;7:e56–68.
Bidot S, Bruce BB. Update on the diagnosis and treatment of idiopathic intracranial hypertension. Semin Neurol. 2015;35:527–38. https://doi.org/10.1055/s-0035-1563569.
Wostyn P, De Deyn PP. The “Ocular Glymphatic System”: an important missing piece in the puzzle of optic disc edema in astronauts? Investig Ophthalmol Vis Sci. 2018;59:2090–1. https://doi.org/10.1167/iovs.17-23263.
Wostyn P, Mader TH, Gibson CR, Killer HE. The escape of retrobulbar cerebrospinal fluid in the astronaut’s eye: mission impossible? Eye. 2019;33:1519–24. https://doi.org/10.1038/s41433-019-0453-8.
Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR. The optic nerve: a new window into cerebrospinal fluid composition? Brain. 2006;129:1027–30. https://doi.org/10.1093/brain/awl045.
Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130:514–20. https://doi.org/10.1093/brain/awl324.
Killer HE, Subramanian PS. Compartmentalized cerebrospinal fluid. Int Ophthalmol Clin. 2014;54:95–102. https://doi.org/10.1097/IIO.0000000000000010.
Killer HE, Jaggi GP, Miller NR, Huber AR, Landolt H, Mironov A, et al. Cerebrospinal fluid dynamics between the basal cisterns and the subarachnoid space of the optic nerve in patients with papilloedema. Br J Ophthalmol. 2011;95:822–7. https://doi.org/10.1136/bjo.2010.189324.
Wostyn P, De Deyn PP. Why space flight-associated neuro-ocular syndrome may differ from idiopathic intracranial hypertension. JAMA Ophthalmol. 2018;136:451–2. https://doi.org/10.1001/jamaophthalmol.2018.0316.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68. https://doi.org/10.1016/j.preteyeres.2009.12.002.
Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–47. https://doi.org/10.1016/j.preteyeres.2008.09.003.
Macias BR, Patel NB, Gibson CR, Samuels BC, Laurie SS, Otto C, et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol. 2020;138:553–9. https://doi.org/10.1001/jamaophthalmol.2020.0673.
Friberg TR. The etiology of choroidal folds. Graefe’s Arch Clin Exp Ophthalmol. 1989;227:459–64. https://doi.org/10.1007/bf02172899.
Mader TH, Gibson CR, Lee AG. Choroidal folds in astronauts. Investig Opthalmol Vis Sci. 2016;57:592. https://doi.org/10.1167/iovs.15-18720.
Wostyn, P, Gibson, CR & Mader, TH Optic nerve sheath stiffness as a predictive biomarker for optic disc edema in astronauts. J Mech Behav Biomed Mater. 2021;124:104846. https://doi.org/10.1016/j.jmbbm.2021.104846 .
Wostyn, P & Nedergaard, M Glymphatic system and Spaceflight Associated Neuro-Ocular Syndrome. In: Lee, AG, Ong, J, editors. Spaceflight Associated Neuro-Ocular Syndrome. Cambridge, MA: Academic Press; 2022. p. 67–76. https://doi.org/10.1016/B978-0-323-91524-3.00016-8.
Wostyn P, Gibson CR, Mader TH. Optic disc edema in astronauts from a choroidal point of view. Aerosp Med Hum Perform. 2022;93:396–8. https://doi.org/10.3357/AMHP.6010.2022.
Kalina RE, Mills RP. Acquired hyperopia with choroidal folds. Ophthalmology. 1980;87:44–50. https://doi.org/10.1016/s0161-6420(80)35279-2.
Comacchio F, Zorzi G, Sacconi R, Laesser R, Pichler A. Increased choroidal thickness in a patient with acquired hyperopia and choroidal folds syndrome. Am J Ophthalmol Case Rep. 2023;29:101803 https://doi.org/10.1016/j.ajoc.2023.101803.
Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40:2583–99. https://doi.org/10.1007/s11064-015-1581-6.
Wostyn P, Killer HE, De Deyn PP. Why a one-way ticket to mars may result in a one-way directional glymphatic flow to the eye. J Neuroophthalmol. 2017;37:462–3. https://doi.org/10.1097/WNO.0000000000000578.
Killer HE, Laeng HR, Groscurth P. Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol. 1999;19:222–8.
Wostyn P, Mader TH, Gibson CR, De Deyn PP. The buffering capacity of the brain and optic nerve against spaceflight-associated neuro-ocular syndrome. Proc Natl Acad Sci USA. 2019;116:15770–1. https://doi.org/10.1073/pnas.1908865116.
Wostyn P, Mader TH, Gibson CR, Killer HE. The perivascular space of the central retinal artery as a potential major cerebrospinal fluid inflow route: implications for optic disc edema in astronauts. Eye. 2020;34:779–80. https://doi.org/10.1038/s41433-019-0594-9.
Wostyn P, De Winne F, Stern C, Mader TH, Gibson CR, De Deyn PP. Potential involvement of the ocular glymphatic system in optic disc edema in astronauts. Aerosp Med Hum Perform. 2020;91:975–7. https://doi.org/10.3357/AMHP.5670.2020.
Galdamez, L. Pathophysiology of cerebral edema and its connection to Spaceflight Associated Neuro-Ocular Syndrome. In: Lee, AG, Ong, J, Editors. Spaceflight Associated Neuro-Ocular Syndrome. Cambridge, MA: Academic Press; 2022. p. 107–133. https://doi.org/10.1016/B978-0-323-91524-3.00002-8.
Galdamez LA, Brunstetter TJ, Lee AG, Tarver WJ. Origins of cerebral edema: implications for spaceflight-associated neuro-ocular syndrome. J Neuroophthalmol. 2020;40:84–91. https://doi.org/10.1097/WNO.0000000000000852.
Fahy, ET, Chrysostomou, V & Crowston, JG Mini-Review: impaired axonal transport and glaucoma. Curr Eye Res. 2016;41:273–83. https://doi.org/10.3109/02713683.2015.1037924.
Hayreh MS, Hayreh SS. Optic disc edema in raised intracranial pressure. I. Evolution and resolution. Arch Ophthalmol. 1977;95:1237–44. https://doi.org/10.1001/archopht.1977.04450070135013.
Hayreh SS. Optic disc edema in raised intracranial pressure. VI. Associated visual disturbances and their pathogenesis. Arch Ophthalmol. 1977;95:1566–79. https://doi.org/10.1001/archopht.1977.04450090088007.
Shen C, Yan S, Du M, Zhao H, Shao L, Hu Y. Assessment of capillary dropout in the superficial retinal capillary plexus by optical coherence tomography angiography in the early stage of diabetic retinopathy. BMC Ophthalmol. 2018;18:113 https://doi.org/10.1186/s12886-018-0778-2.
Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. https://doi.org/10.1016/j.freeradbiomed.2005.03.035.
Takemori K, Murakami T, Kometani T, Ito H. Possible involvement of oxidative stress as a causative factor in blood-brain barrier dysfunction in stroke-prone spontaneously hypertensive rats. Microvasc Res. 2013;90:169–72. https://doi.org/10.1016/j.mvr.2013.08.005.
Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016;36:513–38. https://doi.org/10.1177/0271678X15617172.
Himadri P, Kumari SS, Chitharanjan M, Dhananjay S. Role of oxidative stress and inflammation in hypoxia-induced cerebral edema: a molecular approach. High Alt Med Biol. 2010;11:231–44. https://doi.org/10.1089/ham.2009.1057.
Nusbaum DM, Wu SM, Frankfort BJ. Elevated intracranial pressure causes optic nerve and retinal ganglion cell degeneration in mice. Exp Eye Res. 2015;136:38–44. https://doi.org/10.1016/j.exer.2015.04.014.
Wang Z, Meng CJ, Shen XM, Shu Z, Ma C, Zhu GQ, et al. Potential contribution of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 to blood-brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;48:273–80. https://doi.org/10.1007/s12031-012-9769-6.
Chen S, Chen Y, Xu L, Matei N, Tang J, Feng H. et al. Venous system in acute brain injury: mechanisms of pathophysiological change and function. Exp Neurol. 2015;272:4–10. https://doi.org/10.1016/j.expneurol.2015.03.007.
Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–68. https://doi.org/10.1016/S1474-4422(07)70055-8.
Cherian I, Beltran M, Landi A, Alafaci C, Torregrossa F, Grasso G. Introducing the concept of “CSF-shift edema” in traumatic brain injury. J Neurosci Res. 2018;96:744–52. https://doi.org/10.1002/jnr.24145.
Shin WB, Kim MK, Lee CS, Lee SC, Kim H. Comparison of the clinical manifestations between acute Vogt-Koyanagi-Harada disease and acute bilateral central serous chorioretinopathy. Korean J Ophthalmol. 2015;29:389–95. https://doi.org/10.3341/kjo.2015.29.6.389.
Crucian BE, Zwart SR, Mehta S, Uchakin P, Quiriarte HD, Pierson D. et al. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J Interferon Cytokine Res. 2014;34:778–86. https://doi.org/10.1089/jir.2013.0129.
Crucian B, Stowe RP, Mehta S, Quiriarte H, Pierson D, Sams C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity. 2015;1:15013. https://doi.org/10.1038/npjmgrav.2015.13.
Zwart SR, Gibson CR, Mader TH, Ericson K, Ploutz-Snyder R, Heer M. et al. Vision changes after spaceflight are related to alterations in folate- and vitamin B-12-dependent one-carbon metabolism. J Nutr. 2012;142:427–31. https://doi.org/10.3945/jn.111.154245.
Smith SM, Zwart SR. Spaceflight-related ocular changes: the potential role of genetics, and the potential of B vitamins as a countermeasure. Curr Opin Clin Nutr Metab Care. 2018;21:481–8. https://doi.org/10.1097/MCO.0000000000000510.
Zwart S, Smith S. Genetics, vitamins, and Spaceflight Associated Neuro-Ocular Syndrome. In: Lee, AG, Ong, J, editors. Spaceflight Associated Neuro-Ocular Syndrome. Academic Press; Cambridge, Massachusetts. 2022. https://doi.org/10.1016/B978-0-323-91524-3.00017-X.
Zwart SR, Gregory JF, Zeisel SH, Gibson CR, Mader TH, Kinchen JM. et al. Genotype, B-vitamin status, and androgens affect spaceflight-induced ophthalmic changes. FASEB J. 2016;30:141–8. https://doi.org/10.1096/fj.15-278457.
Kurazumi T, Ogawa Y, Yanagida R, Morisaki H, Iwasaki KI. Non-invasive intracranial pressure estimation during combined exposure to CO(2) and head-down tilt. Aerosp Med Hum Perform. 2018;89:365–70. https://doi.org/10.3357/AMHP.5015.2018.
Law J, Van Baalen M, Foy M, Mason SS, Mendez C, Wear ML. et al. Relationship between carbon dioxide levels and reported headaches on the international space station. J Occup Environ Med. 2014;56:477–83. https://doi.org/10.1097/JOM.0000000000000158.
Lee JK, De Dios Y, Kofman I, Mulavara AP, Bloomberg JJ, Seidler RD. Head down tilt bed rest plus elevated CO2 as a spaceflight analog: effects on cognitive and sensorimotor performance. Front Hum Neurosci. 2019;13:355. https://doi.org/10.3389/fnhum.2019.00355.
Laurie SS, Christian K, Kysar J, Lee S, Lovering AT, Macias BR. et al. Unchanged cerebrovascular CO2 reactivity and hypercapnic ventilatory response during strict head-down tilt bed rest in a mild hypercapnic environment. J Physiol. 2020;598:2491–505. https://doi.org/10.1113/JP279383.
Waisberg E, Ong J, Lee AG. Space radiation and the potential for early cataract development. Eye. 2024;38:416–7. https://doi.org/10.1038/s41433-023-02742-2.
Ong, J & Lee, AG An introduction to space medicine and the physiological effects of spaceflight on the human body. In: Lee, AG, Ong, J, Editors. Spaceflight Associated Neuro-Ocular Syndrome. Cambridge, MA: Academic Press; 2022. p. 1–7. https://doi.org/10.1016/B978-0-323-91524-3.00007-7.
Cucinotta FA, Manuel FK, Jones J, Iszard G, Murrey J, Djojonegro B. et al. Space radiation and cataracts in astronauts. Radiat Res. 2001;156:460–6. https://doi.org/10.1667/0033-7587(2001)156[0460:sracia]2.0.co;2.
Seregard S, Pelayes DE, Singh AD. Radiation therapy: posterior segment complications. Dev Ophthalmol. 2013;52:114–23. https://doi.org/10.1159/000351088.
Barr YR. Lumbar puncture during spaceflight: operational considerations, constraints, concerns, and limitations. Aviat Space Environ Med. 2014;85:1209–13. https://doi.org/10.3357/ASEM.3674.2014.
Hung HL, Kao LY, Huang CC. Ophthalmic features of idiopathic intracranial hypertension. Eye. 2003;17:793–5. https://doi.org/10.1038/sj.eye.6700443.
Patel N, Pass A, Mason S, Gibson CR, Otto C. Optical coherence tomography analysis of the optic nerve head and surrounding structures in long-duration international space station astronauts. JAMA Ophthalmol. 2018;136:193–200. https://doi.org/10.1001/jamaophthalmol.2017.6226.
Bidot S, Bruce BB, Saindane AM, Newman NJ, Biousse V. Asymmetric papilledema in idiopathic intracranial hypertension: response. J Neuroophthalmol. 2015;35:331. https://doi.org/10.1097/WNO.0000000000000284.
Buckey JC, Phillips SD, Anderson AP, Chepko AB, Archambault-Leger V, Gui J. et al. Microgravity-induced ocular changes are related to body weight. Am J Physiol Regul Integr Comp Physiol. 2018;315:R496–R499. https://doi.org/10.1152/ajpregu.00086.2018.
Ong J, Lee AG, Moss HE. Head-down tilt bed rest studies as a terrestrial analog for spaceflight associated neuro-ocular syndrome. Front Neurol. 2021;12:648958. https://doi.org/10.3389/fneur.2021.648958.
Laurie, SS, Vizzeri G, Taibbi G, Ferguson CR, Hu X, Lee S, et al. Effects of short-term mild hypercapnia during head-down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol Rep. 2017;5. https://doi.org/10.14814/phy2.13302.
Laurie SS, Macias BR, Dunn JT, Young M, Stern C, Lee S. et al. Optic disc edema after 30 days of strict head-down tilt bed rest. Ophthalmology. 2019;126:467–8. https://doi.org/10.1016/j.ophtha.2018.09.042.
Globus RK, Morey-Holton E. Hindlimb unloading: rodent analog for microgravity. J Appl Physiol (1985). 2016;120:1196–206. https://doi.org/10.1152/japplphysiol.00997.2015.
Zhang X, Trevino MB, Wang M, Gardell SJ, Ayala JE, Han X. et al. Impaired mitochondrial energetics characterize poor early recovery of muscle mass following hind limb unloading in old mice. J Gerontol A Biol Sci Med Sci. 2018;73:1313–22. https://doi.org/10.1093/gerona/gly051.
Nelson ES, Mulugeta L, Feola A, Raykin J, Myers JG, Samuels BC. et al. The impact of ocular hemodynamics and intracranial pressure on intraocular pressure during acute gravitational changes. J Appl Physiol (1985). 2017;123:352–63. https://doi.org/10.1152/japplphysiol.00102.2017.
Salerni F, Repetto R, Harris A, Pinsky P, Prud'homme C, Szopos M. et al. Biofluid modeling of the coupled eye-brain system and insights into simulated microgravity conditions. PLoS ONE. 2019;14:e0216012. https://doi.org/10.1371/journal.pone.0216012.
Author information
Authors and Affiliations
Contributions
LAG, THM, JO, and AGL wrote the original draft of the manuscript. LAG, THM, JO, CMK, and AGL reviewed and edited the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
AGL is a consultant for the National Aeronautics and Space Administration (NASA) and serves on the editorial board of Eye.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galdamez, L.A., Mader, T.H., Ong, J. et al. A multifactorial, evidence-based analysis of pathophysiology in Spaceflight Associated Neuro-Ocular Syndrome (SANS). Eye 39, 700–709 (2025). https://doi.org/10.1038/s41433-025-03618-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03618-3