Abstract
Despite the wide use of automated devices for the self-measurement of home blood pressure (BP), no evidence is available regarding the accuracy of such devices in oldest-old populations. The aim of this study was to validate the accuracy of the automated oscillometric upper arm-cuff BP-monitoring device according to an international protocol in oldest-old individuals. In 35 participants aged over 85 years old, BP was measured on the same arm sequentially using a mercury sphygmomanometer (by two observers) and an Omron HEM-7080IC. The difference between the test device and observer measurements and associated factors were evaluated according to the International Organization for Standardization (ISO) 81060-2:2013 protocol. A total of 105 pairs (three pairs per participant) of the test device and observer BP measurements were obtained. The mean (±standard deviation: SD) differences in systolic BP (SBP) and diastolic BP (DBP) between the methods were −0.7 ± 7.1 and −1.1 ± 4.5 mmHg, respectively, and those for each participant were −0.7 ± 5.8 mmHg for SBP and −1.1 ± 4.1 mmHg for DBP; the device therefore fulfilled the requirements of the ISO protocol. In the multivariate analysis with the linear mixed model, the difference was associated with the cuff size for SBP and pulse pressure for DBP. The Omron HEM-7080IC passed the ISO requirements for oldest-old individuals aged 85 years or older. This device can be recommended for clinical and self/home use in oldest-old populations.
Similar content being viewed by others
Introduction
The world population is continuing to age rapidly [1]. The older population itself has been aging, with the oldest growing faster than younger demographics. Hypertension increases with age, and approximately 70–80% of oldest-old individuals have hypertension [2, 3]. High blood pressure (BP) is a major risk factor for cardiovascular complications in individuals of all ages [4, 5], and high BP is associated with declining physical function and premature disability in older adults [6,7,8]. While several studies have shown that treatment of hypertension is beneficial for elderly patients, including those aged over 80 years [9, 10], controlling BP in the elderly is challenging because of highly diverse comorbidities, as well as polypharmacy, frailty, cognitive impairment, and variable life expectancy [11].
Self-measurement of BP at home is recommended for older adults because it provides more reliable and reproducible estimations of BP, including minimization of the white-coat effect and the detection of masked hypertension [12]. For the accurate measurement of home BP, the use of validated devices that have been tested for clinical application according to validation standards is mandatory. However, in some special populations, automated devices are problematic, i.e., patients with arterial stiffness [13, 14] and the oldest old in whom vessel stiffness is most frequently encountered [15], and evidence on the accuracy of automated BP devices for the oldest old or elderly populations is very limited [16,17,18]. Therefore, we aimed to investigate the accuracy and reliability of the Omron HEM-7080IC home BP measurement device (Omron Healthcare Co., Ltd., Kyoto, Japan) in individuals older than 85 years according to the International Organization for Standardization (ISO) 81060-2:2013 protocol [19].
Methods
Participants
Outpatients with or without hypertension were recruited from the Department of Geriatric Medicine, Osaka University Hospital (Suita, Japan). We followed the ISO protocol designed for a special population because the oldest-old population was considered to be a possible special population. Since Omron HEM-7080IC uses the same measurement technology as Omron 705-IT, which was validated previously in the general population [20, 21], 35 special population patients were required. A total of 38 patients aged 85 years or older without chronic atrial fibrillation or dementia were screened. Of those, two participants whose BP was unstable and one who could not tolerate repeated measurements were excluded. The number of participants in the present study therefore totaled 35.
The study protocol was approved by the ethics committee of Osaka University Hospital (approval number: 17378). Written informed consent was obtained from each participant.
Device
The Omron HEM-7080IC is an upper-arm electronic oscillometric device with a capacitive pressure sensor designed to measure pressure in the range of 0–299 mmHg and pulse in the range of 40–180 beats per min. Systolic BP (SBP), diastolic BP (DBP), and pulse are shown on a liquid crystal digital display. Inflation is controlled by an automatic pumping system, and deflation is controlled by an automatic pressure release valve. The unit is powered by four alkaline batteries (1.5 V, type LR6), which enable approximately 300 measurements, or by an AC adapter.
A standard cuff can be used for individuals with arm circumferences in the range of 22–32 cm, while a small-sized cuff (arm circumference: 17–22 cm) and large-sized cuff (arm circumference: 32–42 cm) are also available. We measured the arm circumference in individuals so that an adequately sized cuff could be selected.
Procedure
All procedures were conducted in accordance with the ISO protocol [19]. The validation procedure was carried out using same-arm sequential measurements by a team of three medical professional observers who were trained and experienced in BP measurement. Two of the three observers simultaneously measured BP by the auscultatory technique using a standard mercury sphygmomanometer (ACOMA Medical Industry Co., Ltd, Tokyo, Japan), which had been calibrated before the study, and a dual-headed binaural stethoscope with a Y-shaped tube (TSUBASA INDUSTRY Co., Ltd, Tokyo, Japan). The cuffs used for observer measurements were selected such that the bladder dimensions were approximately 80–100% the length/40% the width of each patient’s arm circumference [19, 22]. The observers’ individual measurement values were averaged to determine the observer BP measurements. The third observer was a supervisor who checked the values obtained by the two observers and measured the BP with the test device. The observers were trained previously on how to reach a consensus regarding BP measurements, and each observer was blinded to the other observer’s measurements as well as to the test device measurements throughout the study.
After at least 5 min of rest, the participant sat in a quiet temperature-controlled examination room with his or her arm and back supported, and BP was measured sequentially at the level of the heart on the left arm. Two observers simultaneously measured BP using the mercury sphygmomanometer with the dual-headed binaural stethoscope, followed by measuring BP with the Omron HEM-7080IC as the test device. Neither of these running-in measurements was used for the calculation of the test device accuracy.
Then, BP was measured first by the observers and then by the test device and observers alternately, for a total of seven sequential measurements with at least 1 min between each measurement. Each test-device reading was compared with the mean of the observer measurements taken immediately before and after the test-device measurement; therefore, three pairs of the test device and observer BP measurements were obtained for each participant. According to the ISO protocol [19], we allowed an observer-to-observer difference within 4 mmHg; otherwise, another pair of observer-test BP measurements was taken, with a maximum of eight pairs.
Statistical analysis
Data were analyzed according to criteria 1 and 2 required by the ISO standard [19]. For criterion 1, each test device measurement value was compared with the corresponding observer measurement. The differences between the test device and observer SBP and DBP measurements were calculated using the mean and standard deviation (SD). Bland–Altman plots were used to show deviations in the data [23]. For criterion 2, the mean and SD of each paired BP measurement (test device and observer) was calculated individually for each participant and compared.
We used a linear mixed model that included sex, age, cuff size, upper arm circumference, corresponding observer BP, and pulse pressure to identify confounders causing BP differences. The data were analyzed using SPSS 22.0 for Windows (IBM Japan, Tokyo, Japan). P-values < 0.05 on two-sided tests were considered significant.
Results
The characteristics of the 35 participants are presented in Table 1. Of the participants, 65.7% were women, and the age ranged from 85 to 96 years (87.7 ± 2.6 years). Six participants used a small-sized cuff (a cuff for children: 90-mm-wide cuff bladder was used for BP measurements with a mercury sphygmomanometer), but no one required a large-sized cuff. Three valid determination pairs of the test device and observer BP were obtained, and the ranges of SBP and DBP were 89–180 and 53–97 mmHg for the observer measurements and 77–184 and 44–89 mmHg for the device measurements, respectively. The observer-to-observer differences were 0.0 ± 1.7 mmHg for SBP and 0.0 ± 2.0 mmHg for DBP.
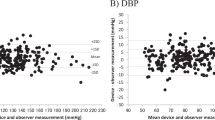
Bland–Altman plots of BP differences between the test device and observer measurements with a total of 105 separate SBP and DBP pairs are presented in Fig. 1. The summary statistics of the differences between observer and device measurements are shown in Table 2. The mean and SD values of the differences between the test device and observer SBP and DBP measurements fulfilled both criterion 1 (−0.7 ± 7.1 mmHg for SBP and −1.1 ± 4.5 mmHg for DBP) and criterion 2 (−0.7 ± 5.8 mmHg for SBP and −1.1 ± 4.1 mmHg for DBP) of the ISO protocol.
Bland–Altman plots of the comparison between the Omron HEM-7080IC device and observer measurements against the mean pressure of the device and observer measurement of the systolic (a) and diastolic (b) blood pressures. Mean differences and 95% limits of agreement between paired measurements are represented by dashed and 2-dot-dashed lines, respectively
In the linear mixed model, the SBP difference between the test device and observer measurements was significantly correlated with the cuff size (P = 0.002, Table 3). The mean device-observer differences were −7.3 ± 6.9 mmHg for the small cuff and 0.7 ± 6.4 mmHg for the standard cuff. However, the DBP difference was associated with pulse pressure (P < 0.001). When divided into three equal subgroups by pulse pressure values, the BP differences of the study participants were all within 5 ± 8 mmHg.
Discussion
To the best of our knowledge, no one has assessed whether home BP can be appropriately measured in an oldest-old population. We assessed the accuracy of the Omron HEM-7080IC device in oldest-old individuals aged 85 years or older according to the ISO 81060–2:2013 protocol. We demonstrated that the device fulfills criterion 1 as well as criterion 2 of the ISO requirements for both SBP and DBP, indicating that the device was accurate and reliable in the oldest-old population.
The increased BP fluctuation in older adults is well known, and BP is easily affected by measurement conditions [24, 25]. Measurement of home BP repeatedly and regularly with unbiased methods is essential regardless of the characteristics of people, including older patients. No study, however, has validated electronic oscillometric monitors used for home BP measurement among an oldest-old population. The Omron HEM-7080IC device uses the same technology as the Omron 705-IT. Coleman A et al. [20] and El Assaad M et al. [21] reported the accuracy of the Omron 705-IT according to the 1993 British Hypertension Society Revised Protocol [26] and the 2002 European Society of Hypertension International Protocol (ESH-IP) [27] among 85 patients aged 47 ± 15 years and among 33 patients aged 54 ± 13 years, respectively. To validate the device in an oldest-old population, we included 35 participants aged 85 years or older in this study according to the requirement of the ISO protocol as a special population. The interdevice differences observed in our study were similar to those observed in the two previous studies of adult populations for both SBP and DBP [20, 21].
In the present study, approximately half of the participants had systolic hypertension, whereas no patients had a DBP of 100 mmHg or higher. Stiffening of the arterial wall and decreasing vessel wall compliance become conspicuous with aging [15]. Such structural changes of the arterial tree increase SBP and decrease DBP, leading to a wider pulse pressure and isolated systolic hypertension as the predominant form of hypertension in older people [28]. The BP distribution in this study would therefore be typical in common oldest-old individuals. Moreover, some previous validation studies excluded patients with isolated systolic hypertension [16, 17], which would be dominantly observed in older populations [28]. The previous ESH-IP [27] specifies three BP categories: low (SBP 90–129/DBP 40–79 mmHg), medium (130–160/80–100 mmHg), and high (161–180/101–130 mmHg). However, the protocol does not clarify, in which BP category patients with isolated systolic hypertension should be placed. The oscillometric method is based on recording arterial wall oscillations, and a greater pulse pressure is associated with a larger oscillometric versus auscultatory BP discrepancy [13, 14]. The probability of incorrect measurement might increase in elderly patients who often show a wider pulse pressure range. In the present study, the differences between the test device and observer measurements were correlated with pulse pressure for DBP measurements. Nevertheless, the BP differences remained within acceptable levels based on the validation criteria. Our findings offer new information on the accuracy of an electronic automated oscillometric BP-measurement device for an oldest-old population in a real-world setting, including those with isolated systolic hypertension.
The multivariate analysis suggested that the cuff size affected the differences in BP measurements between the test device and observer measurements, while the upper arm circumference did not. It has been shown that miscuffing leads to BP under/over estimation [29]. We selected appropriate cuffs for both the test device and reference device following the manufacturer’s instructions and international protocol [19, 22]. Nevertheless, the SBP difference was large among six patients measured with the small cuff; the mean difference was −7.3 ± 6.9 mmHg for the small cuff and 0.7 ± 6.4 mmHg for the standard cuff. Although the measurements satisfied the criteria of the protocol, Stergiou et al. [30] showed reduced accuracy for DBP using a small-sized cuff compared with a standard cuff in a validation study among children and adolescents, which was followed by another validation study in adults [31]. Although a definite conclusion cannot be drawn based on the small number of patients in our study, caution may be required when a small cuff is applied in an oldest-old population. To date, few previous validation studies targeting adults have included participants with slender arms who require a small cuff [31, 32]. Further studies are needed to clarify the accuracy of the oscillometric devices in individuals who require a small cuff. Furthermore, we did not have a patient with an arm thickness of 32 cm or more who required a large-sized cuff. Japanese patients generally have slender arms. Japanese anthropometric reference data reported that the median (10th−90th percentile) arm circumference in individuals over 85 years old is 24.0 (19.4–28.4) cm in men and 22.6 (18.2–27.9) cm in women [33]. While there are some countries or regions where it may be rare to use a large-sized cuff [34, 35], in other countries such as the United States of America, there are a number of patients with thick arms who need a large-sized cuff [36, 37]. For example, the 75th percentile value of arm circumference in those aged 80 years or older in the U.S.A. is 31.9 cm for females and 33.0 cm for males; a quarter of older people need a large-sized cuff [36].
Participants with chronic atrial fibrillation or any sustained arrhythmia were not included in this study. Frequent ectopic beats could affect the accuracy of both auscultatory and oscillometric techniques as a result of increased beat-to-beat BP variability. Although atrial fibrillation is more prevalent in older people than younger adults, there is currently no agreed-upon procedure for BP monitor validation in patients with such conditions [22].
Self-measurement of BP at home is recommended for older adults. We showed that the Omron HEM-7080IC can be recommended for self/home use in the oldest-old population, although caution may be required when a small cuff is applied. However, oldest-old individuals have heterogeneous patterns of comorbidities and severity of conditions [11]. In a clinical setting, medical professionals need to take into account the patient’s condition, such as cognitive function and activities of daily living, when applying self-measurement of home BP to oldest-old individuals. The issue regarding which of the measured home BP values should be used for clinical evaluation also needs to be considered [38, 39].
In conclusion, this study is the first to report that automated electronic devices for BP monitoring are able to accurately and reliably measure BP in a special population of oldest-old individuals. We can expand the application of self-measurement of home BP to oldest-old populations, although some considerations are required.
References
He W, Goodkind D, Kowal P, US Census Bureau. An aging world: 2015, International Population Reports. Washington, DC: U.S. Government Publishing Office; 2016. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95-16-1.pdf. Accessed 17 Apr 2019.
McDonald M, Hertz RP, Unger AN, Lustik MB. Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among United States adults aged 65 and older. J Gerontol A Biol Sci Med Sci. 2009;64:256–63.
Ishine M, Okumiya K, Hirosaki M, Sakamoto R, Fujisawa M, Hotta N, et al. Prevalence of hypertension and its awareness, treatment, and satisfactory control through treatment in elderly Japanese. J Am Geriatr Soc. 2008;56:374–5.
Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–72.
Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, et al. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res. 2012;35:947–53.
Dumurgier J, Elbaz A, Dufouil C, Tavernier B, Tzourio C. Hypertension and lower walking speed in the elderly: the Three-City study. J Hypertens. 2010;28:1506–14.
Hajjar I, Lackland DT, Cupples LA, Lipsitz LA. Association between concurrent and remote blood pressure and disability in older adults. Hypertension. 2007;50:1026–32.
Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354:971–5.
Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98.
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16.
American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1–E25.
Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29.
van Popele NM, Bos WJ, de Beer NA, van Der Kuip DA, Hofman A, Grobbee DE, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484–8.
Pannarale G, Bebb G, Clark S, Sullivan A, Foster C, Coats AJ. Bias and variability in blood pressure measurement with ambulatory records. Hypertension. 1993;22:591–8.
O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8.
Altunkan S, Iliman N, Altunkan E. Validation of the Omron M6 (HEM-7001-E) upper arm blood pressure measuring device according to the International Protocol in elderly patients. Blood Press Monit. 2008;13:117–22.
Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Press Monit. 2007;12:233–42.
Rosholm JU, Arnspang S, Matzen L, Jacobsen IA. Auscultatory versus oscillometric measurement of blood pressure in octogenarians. Blood Press. 2012;21:269–72.
ISO 81060-2:2013, Non-invasive Sphygmomanometers—Part 2: Clinical Investigation of Automated Measurement Type. International Organization for Standardization. 2013.
Coleman A, Freeman P, Steel S, Shennan A. Validation of the Omron 705IT (HEM-759-E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit. 2006;11:27–32.
El Assaad MA, Topouchian JA, Asmar RG. Evaluation of two devices for self-measurement of blood pressure according to the international protocol: the Omron M5-I and the Omron 705IT. Blood Press Monit. 2003;8:127–33.
Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36:472–8.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Kamide K, Kabayama M. Implications of blood pressure variations in older populations. Hypertens Res. 2019;42:19–25.
Jansen RW, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med. 1995;122:286–95.
O’Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, Altman DG, et al. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11:677–9.
O’Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7:3–17.
Thijs L, Den Hond E, Nawrot T, Staessen JA. Prevalence, pathophysiology and treatment of isolated systolic hypertension in the elderly. Expert Rev Cardiovasc Ther. 2004;5:761–9.
O’Brien E. Review: a century of confusion; which bladder for accurate blood pressure measurement? J Hum Hypertens. 1996;10:565–72.
Stergiou GS, Yiannes NG, Rarra VC. Validation of the Omron 705 IT oscillometric device for home blood pressure measurement in children and adolescents: the Arsakion School Study. Blood Press Monit. 2006;11:229–34.
Alpert BS. Validation of the Omron HEM-9210T by the ANSI/AAMI/ISO 81060-2: 2013 with two novel cuffs: wide-range and extra-large. Blood Press Monit. 2017;22:166–8.
Bing S, Chen K, Hou H, Zhang W, Li L, Wei J, et al. Validation of the Microlife BP A200 Comfort and W2 Slim automated blood pressure monitors in a general adult population according to the European Society of Hypertension and the ANSI/AAMI/ISO 81060-2: 2013 protocols. Blood Press Monit. 2016;21:118–23.
Hosoya N, Okada T, Muto Y. Japanese Anthropometric reference data: JARD 2001 (in Japanese). Jpn J Nutr Assess. 2002;19(Suppl):1–81.
Gavriilidou NN, Pihlsgard M, Elmstahl S. Anthropometric reference data for elderly Swedes and its disease-related pattern. Eur J Clin Nutr. 2015;69:1066–75.
Bannerman E, Reilly JJ, MacLennan WJ, Kirk T, Pender F. Evaluation of validity of British anthropometric reference data for assessing nutritional state of elderly people in Edinburgh: cross sectional study. BMJ. 1997;315:338–41.
Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2011–4. Vital Health Stat 3. 2016;39:1–46.
Barbosa AR, Souza JM, Lebrão ML, Laurenti R, Marucci Mde F. Anthropometry of elderly residents in the city of São Paulo, Brazil. Cad Saude Publica. 2005;21:1929–38.
Imai Y, Obara T, Ohkubo T. How many times should we ask subjects to measure blood pressure at home on each occasion? J Hypertens. 2007;25:1987–91.
Rhee MY, Kim JY, Kim JH, Namgung J, Lee SY, Cho DK, et al. Optimal schedule of home blood-pressure measurements for the diagnosis of hypertension. Hypertens Res. 2018;41:738–47.
Acknowledgements
This study was supported by a research grant from Omron Healthcare Co., Ltd. We thank all patients who participated to this study. We would like to thank Drs. Takeya, Kurinami, and Akasaka from the Department of Geriatric Medicine, Osaka University Hospital for their cooperation with this study. We also thank Ms. Tuo, Mr. Akagi, and other members of the Health Science Division, Osaka University Graduate School of Medicine for their assistance in conducting this study.
Funding
KA, KK, and TO recieved research grants from Omron Healthcare Co., Ltd. The sponsor of this study was not involved in data collection or analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KSa is a salaried employee of Omron Healthcare Co., Ltd. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Godai, K., Kabayama, M., Saito, K. et al. Validation of an automated home blood pressure measurement device in oldest-old populations. Hypertens Res 43, 30–35 (2020). https://doi.org/10.1038/s41440-019-0330-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-019-0330-7
Keywords
This article is cited by
-
Clinical validation of a wearable ultrasound sensor of blood pressure
Nature Biomedical Engineering (2024)
-
In-office and out-of-office blood pressure measurement
Journal of Human Hypertension (2021)
-
Recent status of self-measured home blood pressure in the Japanese general population: a modern database on self-measured home blood pressure (MDAS)
Hypertension Research (2020)
-
Frailty and hypertension in older adults: current understanding and future perspectives
Hypertension Research (2020)
-
Day-to-day blood pressure variability is associated with lower cognitive performance among the Japanese community-dwelling oldest-old population: the SONIC study
Hypertension Research (2020)