Abstract
Worldwide, hypertension and chronic kidney disease (CKD) are highly prevalent disorders and are strong risk factors for cardiovascular disease and end-stage renal disease (ESRD). The developmental origins of health and disease (DOHAD) concept suggests that undesirable perinatal environmental conditions, such as malnutrition, contribute to disease development in adults. Among the known hypertension and CKD risk factors, DOHAD plays a potential role in determining susceptibility to the onset of these diseases in later adulthood. Since low birth weight (LBW) is a surrogate marker for adverse fetal environmental conditions, the high incidence of LBW in developing countries and its increasing incidence in most developed countries (attributed to multiple pregnancies and prepregnancy maternal factors, such as undernutrition, advanced maternal age, and smoking) is concerning. Thus, LBW is an important public health problem not only because of the associated infant mortality and morbidity but also because it is a risk factor for adult-onset hypertension/CKD. During their reproductive years, pregnant women who were born with LBWs have an increased risk of hypertensive disorders of pregnancy, which contribute to the risk of developing cardiovascular disease and ESRD. The offspring of LBW females are also likely to be LBW, which suggests that susceptibility to hypertension/CKD may reflect transgenerational inheritance. Therefore, there is global concern about the increasing prevalence of LBW-related diseases. This review summarizes the relevance of hypertension and CKD in conjunction with DOHAD and discusses recent studies that have examined the impact of the upward LBW trend on renal function and blood pressure.
Similar content being viewed by others
Introduction
Hypertension (HTN) is a common disorder that affects 25–35% of adults worldwide [1]. Although several treatments have been developed, the control of HTN remains insufficient [2]. Recently, the global prevalence of chronic kidney disease (CKD) has been determined to be 13.4% and is rising [3, 4]. Furthermore, CKD is the fourth fastest increasing cause of death globally [5]. Both CKD and HTN are strong risk factors for cardiovascular disease and end-stage renal disease (ESRD), with ESRD having a particularly great economic impact on social systems due to the high cost of renal replacement therapy [6]. Preemptive medical treatment is a strategy for managing these disorders [7], and the developmental origins of health and disease (DOHAD) concept has been recognized as a potential source of clues for preventing these diseases [8].
Low birth weight (LBW) is regarded as an indicator of intrauterine growth restriction (IUGR) and/or preterm birth (<37 weeks gestation). LBW is believed to be an antecedent of chronic noncommunicable diseases (NCDs) and a potential contributor to HTN and CKD in adults. The prevalence of LBW is high in developing countries and is increasing in most developed countries [9, 10], making it a global public health problem associated with mortality and morbidity in infants and adults. However, the actual influence of the increasing trend in LBW prevalence on renal function and blood pressure (BP) is currently unknown. In this review, we examine the recent evidence for the effects of LBW on renal function/BP and discuss the impact of the increasing prevalence of LBW on the incidence of CKD/HTN.
LBW prevalence
LBW, defined as a birth weight ≤2500 g, is attributed to IUGR (small for gestational age) or preterm birth (birth before 37 weeks of pregnancy) and has a prevalence of ~10–15% in developing countries [10]. In addition, the prevalence of LBW in most countries belonging to the Organization for Economic Cooperation and Development has been increasing since 1990. On average, in these countries, LBW births have a prevalence of 6.5% among live births [11]. The reasons for the global increase in LBW prevalence include increases in the number of multiple pregnancies (probably due to reproductive medicine improvements), maternal prepregnancy factors (e.g., older ages at parturition and undernutrition), and increased smoking by young women [12]. Increases in the numbers of LBW infants, as a proportion of total live births, are also high in East Asia, including in Japan and Korea [11]. In Japan, the prevalence of LBW has been increasing since the 1980s, and the current prevalence is high (9.4%), even when compared with the average for countries in the Organization for Economic Cooperation and Development (6.5%) [11]. In Japan, strict body weight management during pregnancy had been recommended for a long time, and a large percentage of young women remain underweight [13]. In Korea, the proportion of LBW births has increased along with the proportion of underweight young women [14]. Recently, ~25% of young women in Japan and ~15% of young women in Korea are underweight (BMI <18.5), compared with ~2% of young women in the USA. Therefore, as seen in developing countries, malnutrition in young women might contribute to the increasing prevalence of LBW births. This upward trend in the numbers of LBW births has raised concern about the potential influence of LBW on NCD development [15].
Impacts of increased LBWs on renal function
The actual effect of declining average birth weight on NCD development remains unknown. To investigate the relationship between the upward trend in LBW prevalence and renal function, we conducted an annual cross-sectional study over an 18-year period (1998–2015), which included 3737 male adolescents who did not manifest age-related CKD [16]. We found that both renal function and birth weight have decreased since 1998 in these apparently healthy adolescents. Although metabolic parameters, such as serum glucose and total cholesterol, were not associated with reduced renal function, a significant association between stage 2 CKD and LBW was found. To the best of our knowledge, this was the first study to demonstrate an actual association between the trend of LBW and renal function. We also reported a significant increase in the prevalence of proteinuria in the same study of adolescent males [16]. According to a national survey, the prevalence of proteinuria in high school students in Japan has gradually increased in conjunction with decreasing birth weights since the 2000s (Fig. 1) [13, 17].
DOHAD
The DOHAD concept suggests that undesirable environmental conditions, such as maternal malnutrition and smoking during the perinatal period, influence the susceptibility to NCDs during adulthood. For several decades, epidemiological studies have pointed to an association between insults occurring in utero and during infancy with NCD risk. In the 1970s, individuals who had been exposed as fetuses to the Dutch winter famine during World War II were reported to frequently manifest lifestyle-related diseases, such as adulthood obesity [18, 19], implying the potential contribution of malnutrition during pregnancy to the future onset of disease. In the 1980s, Barker et al. reported a geographic relationship between high cardiovascular mortality and high infant mortality in England and Wales, where social conditions were poor and death rates have been consistently elevated [20]. They also found that LBW individuals have higher incidences of ischemic heart disease, HTN, and insulin resistance [21]. Based on these findings, the same authors also proposed that adulthood NCDs may originate from fetal malnutrition, the so-called “fetal origins of adult disease (FOAD) theory” [22]. In the 1990s, animal experiments documented the phenotype of fetal programming of NCDs. Entering this century, investigations into the possible underlying mechanisms of FOAD progressed, with the FOAD hypothesis developing into the broader concept of DOHAD [23]. The DOHAD concept suggests that the period from the early embryonic stage through childhood is important for determining NCD susceptibility later in life.
CKD and the DOHAD concept
The main causes of CKD include diabetes, HTN, and glomerulonephritis, while aging also contributes to its development [24]. LBW has also been identified as a potential risk factor for CKD. Reduced renal function, evaluated using the estimated glomerular filtration rate (eGFR), has been documented in adolescents who were LBW babies [25], and their renal function likely declines with advancing age [24]. Furthermore, in young adulthood, LBW individuals have been found to have decreased renal function, as assessed using creatinine clearance (the odds ratio [OR] for low–normal creatinine clearance (<100 ml/min) in young adults who were LBW babies was 1.66, with a 95% confidence interval [CI] of 1.16–2.37) [26]. In later life, meta-analyses further demonstrated a significant relationship between LBW and the onset of CKD, defined as albuminuria, reduced eGFR (<60 ml/min/1.73 m2 or <10th centile for age/sex), or ESRD (OR, 1.73; 95% CI, 1.44–2.08) [27]. A recent meta-analysis found that LBW contributes to a 70% increased risk of CKD in adulthood; the risk was especially high among Asian and Australian populations (reduced eGFR (<60 ml/min/1.73 m2): OR, 2.68; 95% CI, 1.73–4.15; albuminuria: OR, 2.28; 95% CI, 1.17–4.43) [28]. For every 1 kg increase in birth weight, the eGFR (calculated using creatinine and cystatin-based formulas) increased by 2.13 ml/min/1.73 m2 (95% CI, 0.69–3.58) [29].
Preterm birth, regardless of birth weight, is also a risk factor for the development of CKD in mid-adulthood; preterm individuals have a twofold higher risk of CKD than do individuals born at full term [30]. Pathological assessments showed that LBW and preterm birth are also risk factors for the development of secondary focal segmental glomerulosclerosis (FSGS) [31, 32]. The glomerulomegaly, focal and segmental matrix expansion, and podocytopenia seen in these patients might contribute to the progression of proteinuria and FSGS [31]. As also shown for diabetic kidney disease [33], individuals with LBW are prone to various primary and secondary renal diseases [32, 34, 35]. Together, these data indicate that LBW is associated with an increased susceptibility to renal disease that can, in turn, contribute to CKD progression.
HTN and the DOHAD concept
As in reports regarding CKD, LBW is also associated with high BP in later life. Specifically, LBW was significantly associated with a 30% increased risk of HTN compared with term infants with normal birth weights (OR, 1.30; 95% CI, 1.16–1.46) [36]. Furthermore, a significant inverse correlation between BP and birth weight was observed; for every 1 kg increase in birth weight, systolic BP was reduced by 1.36 mmHg (95% CI, −1.62 to −1.09), and diastolic BP was reduced by 0.33 mmHg (95% CI, −0.54 to −0.13) [36]. In a study involving twins, the twin with the lower birth weight manifested a more rapid increase in systolic BP during infancy [37], indicating that prenatal environmental factors determine susceptibility to high BP, regardless of genotype. In addition, African–Americans often demonstrate higher BPs than White Americans, and IUGR can explain the racial predisposition to high BP. Birth weights are often lower in Black infants than in White infants, and BPs are typically higher in Black adolescents than in White adolescents, indicating that intrauterine growth may play an important role in the Black/White difference in BPs [38]. Preterm birth, regardless of birth weight, is also a risk factor for HTN development. Individuals born preterm are more likely to have higher BPs (on average, 2.5 mmHg higher) than individuals born at term [39]. Furthermore, individuals born preterm remain smaller than their full-term counterparts; however, any postnatal catch-up growth augments the risk of elevated BP [40]; therefore, the perinatal period plays an important role in regulating BP in later life. In our 18-year study, we did not observe increased BPs in adolescents with LBWs, nor was there a significant upward trend in BP values [16, 41]. Accordingly, the association between LBW and the development of HTN in adolescence is inconsistent among several reports [16, 41,42,43]. Young adults who had very LBWs (VLBWs; birth weight <1500 g) showed increased BPs compared with others born at term [44]. Young adults with VLBWs demonstrated 24-h systolic BPs that were 2.4 mmHg higher (95% CI, 0.2–4.6) after adjusting for sex, age, and body mass index (BMI) than for those born with normal birth weights [45]. Even among those born with VLBWs, the apparent increase in mean BP is small; therefore, the increased BPs are only evident in later adulthood, as confirmed by epidemiological studies [46]. Individuals with LBWs are vulnerable to obesity, and BMI is likely a potential contributor to increased BP, especially in children and adolescents.
DOHAD mechanisms
Although several potential mechanisms for DOHAD have been suggested, there are likely two main mechanisms. One is that immature organ development may be induced by gestational stress, which leads to structural changes and impaired functioning of the affected organ. For example, LBW is a risk factor for diabetes in adults, and reduced islet cell mass and β cell numbers have been reported in LBW infants [47]. Another possible mechanism is epigenetic modification. Below, we summarize the possible mechanisms for disturbances in renal function and BP in LBW individuals.
Low nephron numbers
A low number of nephrons is a major, known factor that determines the development of CKD and HTN (reviewed in [48]). Nephron numbers decrease linearly as individuals age, at least until the age of 70 years [49]. LBW due to IUGR and/or preterm birth affects renal function by causing a reduction in the number of functional nephrons, as suggested by Brenner et al. [50, 51]. The fetal period is a critical period for nephron formation (nephron endowment), and nephron formation continues until 36 weeks gestation; additional nephrons do not develop thereafter [52]. An analysis of autopsies performed on IUGR infants showed a linear increase in the number of glomeruli as the birth weight rose to 3000 g, after which the number stabilized [53]. In preterm infants, nephrogenesis continues after birth, but preterm infants have fewer functional nephrons [54]. The reduction in the number of glomeruli observed in IUGR/preterm births decreases the surface area available for filtration, and the development of glomerular hyperfiltration could eventually cause proteinuria and glomerular sclerosis. In turn, increases in proteinuria and glomerular sclerosis may lead to further glomerular hyperfiltration. Ethnic groups that are prone to renal disease, including aboriginal Australians, have low nephron numbers and large glomeruli and are at an increased risk of developing ESRD [55]. Overall, nephron numbers vary widely between ethnic groups, with Japanese people having a particularly low number of nephrons, probably due to their short stature, compared with other ethnicities [56]. Furthermore, the low nephron numbers seen in Asians may account for their high incidence of ESRD [6].
The total GFR is the product of the total nephron number and the single-nephron GFR. Since the single-nephron GFR is stable in healthy adults, the decreasing number of nephrons that has been associated with aging is believed to lead to a decreasing total GFR [49]. According to the glomerular hyperfiltration theory, the single-nephron GFR is likely to be higher in LBW individuals. Indeed, we and others have demonstrated that adolescents born with LBWs have lower eGFRs than adolescents born with normal birth weights [16, 25]. These results do not necessarily indicate that the single-nephron GFR is increased to compensate for the low nephron numbers in such individuals. The significance of the single-nephron GFR on LBW-related kidney disease could be more evident after secondary insults, such as obesity and HTN, which also cause deterioration of glomerular hyperfiltration. Brenner et al. proposed that low nephron numbers may reduce renal salt excretion because of the reduced filtration surface area, increasing an individual’s susceptibility to high BP in the setting of high sodium intake. In fact, LBW individuals do manifest increased salt sensitivity [57], which results from shifting pressure natriuresis curves and the maintenance of high BP.
Cardiovascular development
IUGR and preterm birth affect proper vascular development and result in vascular structure and function alterations. During microvascular development, the reduced density of arterioles/capillaries and the narrowing of arterioles, both observed in individuals with LBW, lead to increased peripheral vascular resistance that can contribute to increased BP [58, 59]. Macrovascular development is also affected by LBW. The production of elastin, an extracellular matrix protein, is largely restricted before birth and is also reduced in LBW individuals; reduced elastin production leads to aortic stiffness and high BP [60]. These vascular structural alterations affect the functioning of the vascular system, including a contribution to the endothelial dysfunction observed in adults born with LBW [61, 62]. In addition to the effect on BP, insufficient vascular development may affect renal function [63]. LBW mice, the development of which is induced through prenatal caloric and protein restriction, manifest low renal vascular densities and endothelial dysfunction, which contribute to declines in renal blood flow and GFR [64]. Impaired cardiovascular structure and function have also been reported in cases of severe, acute malnutrition during childhood [65]. Such individuals manifest increased peripheral vascular resistance at ~30 years of age, suggesting that they will be hypertensive later in life [65].
Epigenetic modifications
Epigenetic modifications (i.e., altered gene expression without DNA sequence changes) have been suggested as potential molecular mechanisms of DOHAD [66]. For example, promoter hypomethylation is associated with transcriptional permissiveness that results in increased target gene expression. Among survivors of the Dutch winter famine, malnutrition (maternal intake of 400–800 calories/day during pregnancy) has been associated with an increased risk of NCD development once the affected fetuses reached adulthood [19]. Fetal exposure to famine led to hypomethylation of insulin-like growth factor 2, which might influence growth and development [67], and altered the methylation status of several other genes [68]. Hypomethylation of the angiotensin-converting enzyme gene promoter [69] and the Ang II receptor type 1 receptor in the adrenals [70] was reported in protein-restricted animal models to lead to HTN, as suggested by the DOHAD concept. The role of epigenetic mechanisms in the development of CKD remains under investigation [71]; some studies have reported the participation of the epigenome in the development of CKD induced by LBW. Recently, Wanner et al. demonstrated that DNA methyltransferases are abundant during nephrogenesis and that DNA methyltransferase 1 deficiency induces global DNA hypomethylation in kidney progenitor cells and upregulates genes such as germline genes, leading to reduced differentiation of the nephrons [72]. These changes reduce nephron numbers and lead to renal hypoplasia at birth, indicating the role of DNA methylation in renal development, especially as related to IUGR [72].
Other factors
LBW is also associated with sympathetic nerve activation, which may explain BP increases [73, 74]; experimental data have shown that sympathetic nerve activity is increased in a rat model with IUGR [75]. Gene expression changes in components of the renin–angiotensin system and in renal sodium transporters have also been considered to increase BP in this model (reviewed in [76]). Recently, the gut microbiota has been associated with NCDs, and gut microbiota alterations are believed to contribute to BP regulation through changes in the composition of the gut microbiota and/or its metabolites, such as short-chain fatty acids and trimethylamine N-oxide (reviewed in [77]). For example, short-chain fatty acids were shown to stimulate renin secretion and regulate BP through the Olfr78 and Gpr41 receptors [78]. Furthermore, transgenerational inheritance of high BP has been suggested to be related to the gut microbiota [77]. Some studies have also demonstrated that the gut microbiota is altered in pregnant hypertensive patients [79]; this implies that the gut microbiota acquired from an individual’s mother might contribute to HTN. However, some LBW infants are delivered by cesarean section and may avoid exposure to the maternal gut microbiota. Thus, the role of early colonization on NCD development has not been clearly elucidated. The strain composition of the gut microflora remains stable for several years [80], suggesting that the gut microbiota acquired during the fetal period may affect susceptibility to HTN/CKD when the individual reaches adulthood.
Sex impact on CKD in the DOHAD concept
Previous studies investigating sex differences have revealed that females have significantly lower nephron numbers than males (794,493 ± 248,725 vs. 941,023 ± 337,705), suggesting an increased risk of CKD in females based on a reduced renal endowment [81]. However, one meta-analysis showed that renal functioning declines faster among males than among females, despite females having a lower nephron number [82]. Theoretically, a protective effect of estrogen in females and higher risk lifestyles among males might explain the faster renal functioning decline in males [83]. To date, some reports demonstrate that the potential association between birth weight and CKD is weak in women [84]. To determine sex differences in CKD susceptibility due to birth weight, we evaluated the annual cross-sectional data from 2417 Japanese adolescents (1736 males and 681 females), 15–16 years old, over an 8-year period (2007–2014) [41]. We noted that birth weights and eGFRs of the study subjects decreased over the course of our study; LBW was significantly associated with renal dysfunction in both male and female adolescents. The incidence of stage 2 CKD was significantly related to LBW (males: OR, 1.73; 95% CI, 1.06–2.80; females: OR, 3.29; 95% CI, 1.25–8.02), although the accuracy may have been lost when determining the higher GFR because we used an equation to estimate GFR for Japanese adolescents with CKD [16, 85]. These results indicate that LBW individuals of either sex may be vulnerable to CKD in later life.
We also considered the effects of secondary insults, such as catch-up growth and later obesity, on the occurrence of HTN/CKD. In developing countries, a high LBW prevalence and subsequent obesity, due to poor nutrition, synergistically contribute to NCD development [10]. In Japan, the prevalence of obesity is increasing among men but not women [86]. In fact, the mean body weight of young Japanese women is low, despite the global trend toward obesity. In view of the recent increases in LBW prevalence and subsequent obesity later in life, we speculate that the incidence of CKD in men might also increase, as previously suggested [87]. The high percentage of underweight women, the pregnancy-related increase in BP, and the deterioration of renal function (discussed below) indicate the need for a longitudinal study to evaluate the effects of LBW on renal function and BP.
Pregnancy and the DOHAD concept
Pregnancy unmasks a vulnerable female to LBW-related diseases. Western diets, lack of exercise, and aging are factors that exacerbate LBW-related NCDs; however, pregnancy is another of these factors for reproductive-age women. During the early stages of pregnancy, women manifest small BP decreases due to reduced peripheral vascular tone. As pregnancy progresses, BP normalizes, and blood volume is expanded with an increased GFR [88]. This hypervolemic state further exacerbates HTN and proteinuria, especially in individuals with poor reserves, such as those with low nephron numbers or vascular remodeling. Interestingly, there is a significant correlation between a pregnant woman’s own birth weight and the hypertensive disorders of pregnancy/renal dysfunction that she may experience. According to a previous study, the ORs for the development of HTN in a female born following IUGR or born preterm were 1.8 (95% CI, 1.1–2.8) and 1.5 (95% CI, 0.96–2.5), respectively [89]. LBW and preterm birth were significantly related to an increased risk of preeclampsia (birth weight <2041 g: OR for preeclampsia, 2.19 (95% CI, 0.91–5.25); prematurity: OR, 1.39 (95% CI, 1.01–1.93)) [90]. In addition, females with previous preeclampsia have higher risks of developing ESRD and HTN than females without it [91]. Therefore, LBW females who experience pregnancy-related complications require long-term follow-up to prevent CKD and HTN.
Transgenerational effects of DOHAD
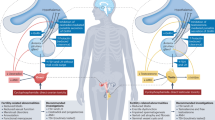
Adverse environmental conditions in utero or during infancy affect not only a parent’s susceptibility to NCDs but also that of their offspring [67, 92, 93]. According to observational studies involving survivors of the Dutch winter famine, malnutrition during pregnancy has life-long effects on the health of affected fetuses that reach across generations [67]. Two possible mechanisms for the transgenerational effects of DOHAD have been suggested (reviewed in [94]). First, as shown in a previous paragraph, LBW females have an increased risk for hypertensive disorders during pregnancy. These disorders can also retard the fetal growth of their offspring, resulting in another generation of LBW children [95]. Second, undesirable uterine conditions also affect epigenetics by inducing permanent gene expression changes in germ cells. Several animal experiments have revealed that malnutrition during pregnancy contributes to increased BPs in the offspring once they reach adulthood [94, 96]. In humans, supporting evidence for the transgenerational effects of DOHAD has also been reported. The risk of preeclampsia and gestational HTN is increased in individuals born with LBW, and these hypertensive disorders are associated with the next generation of offspring having LBWs and an increased risk of HTN in later life [97]. Systolic BP values are higher in the offspring of mothers who experienced hypertensive disorders of pregnancy than in the offspring of mothers without such disorders (gestational HTN: 2.06 mmHg; 95% CI, 1.28–2.84; preeclampsia: 1.12 mmHg; 95% CI, 0.89–3.12). These hypertensive disorders are associated with changes in cord blood DNA methylation patterns in the offspring, which may contribute to HTN onset in the offspring [98]. In Japan, birth weights have been decreasing since the 1980s, and LBW females have now reached reproductive ages. Thus, close attention should be paid to the health conditions of both the mothers and their offspring. The concept of DOHAD impacting the onset of HTN/CKD is summarized in Fig. 2.
The impact of life-course insults on HTN/CKD occurrence as related to the concept of DOHAD. LBW is an indicator of undesirable perinatal environmental conditions and predisposes to the occurrence of HTN/CKD. Secondary insults such as obesity and pregnancy add to the risk during the life course. HDP hypertensive disorder of pregnancy, LBW low birth weight, CKD chronic kidney disease, HTN hypertension
Management of CKD and HTN relative to DOHAD
The effective treatment of CKD and HTN in adults remains insufficient to combat the global increases in the prevalence of these diseases, making the early recognition of risk factors and developing prevention strategies especially important. The management of CKD and HTN within the DOHAD concept was reviewed by the Low Birth Weight and Nephron Number Working Group [99]. They determined that medical histories should include birth weights for patients of all ages to screen for high-risk (LBW) patients [100]. In particular, individuals born prematurely and/or with VLBWs are vulnerable to acute kidney injury due to nephrotoxic agents with the presence of incomplete nephrogenesis. Indeed, acute kidney injuries occur in 40% of such individuals during infancy [101], and these injuries are potential risk factors for subsequent CKD development [102]. Therefore, medical histories should also include information regarding neonatal acute kidney injury.
Prenatal calorie restriction, followed by postnatal overfeeding, also contributes to an increased NCD risk [103, 104]. This association between IUGR and subsequent “catch-up” (glucose- and fat-enriched) diets and NCDs was demonstrated in animal models [105]. Furthermore, accelerated kidney cell senescence, following catch-up growth, was observed in LBW rats [106]. A meta-analysis demonstrated that young adults who were born preterm have higher (~3 mmHg) systolic BPs than individuals born at term [107]. As BP is known to track with age [108], these small differences between preterm and term individuals are likely to increase with age. Young adults with prehypertension already have an increased risk of developing cardiovascular disease [109]; therefore, early treatment involving lifestyle modifications and/or antihypertensive medication could theoretically prevent the onset of HTN and CKD [107].
Treatment with renin–angiotensin blockade
The activity of the renin–angiotensin II-aldosterone system is high in individuals with LBW-related renal complications and HTN. In LBW rat pups, the intrarenal renin–angiotensin system is upregulated by four weeks of age, unlike in control animals [110]. This upregulation leads to increased efferent arteriolar resistance and glomerular HTN. Pathological assessments have also shown that VLBW, LBW, and premature birth are risk factors for secondary FSGS [31, 32], for which blocking of the renin–angiotensin system may reduce proteinuria and retard renal injury. Indeed, a case report has shown that treatment with an angiotensin-converting enzyme inhibitor effectively ameliorates proteinuria and HTN in patients born extremely prematurely [111]. In addition, patients with both autosomal dominant polycystic kidney disease and LBW benefit more from the use of drugs that block the renin–angiotensin system compared with the use of beta-blockers or diuretics, as these drugs postpone the onset of ESRD by 4.3 years [112]. Therefore, the use of angiotensin-blocking agents in patients with LBW-related renal complications may be reasonable.
Conclusion
The prevention and treatment of various age-related NCDs are becoming crucial issues as the number of elderly people increases, especially in Japan. Preemptive medical treatments comprise a potential effective strategy for combatting NCDs because they intervene before disease onset to actively prevent the disease. The relevance of the DOHAD concept has been recognized because of its impact on our understanding of HTN/CKD development, particularly in view of the increasing prevalence of LBW births worldwide. In Japan, the mean annual eGFR has decreased and the incidence of proteinuria has increased over the last two decades, and these changes are significantly associated with the increased number of Japanese adolescents who were LBW babies [16, 41]. These results indicate that adolescents who had LBWs are prone to HTN and CKD during their later lives. Considering the risk of HTN/CKD onset, establishing a screening and follow-up program for managing individuals who were born with LBWs and their offspring is needed.
References
Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–41.
Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015;65:54–61.
Coresh J. Update on the Burden of CKD. J Am Soc Nephrol. 2017;28:1020–2.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE. 2016;11:e0158765.
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. https://doi.org/10.1016/S0140-6736(14)61682-2.
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–82.
Itoh H, Hayashi K, Miyashita K. Pre-emptive medicine for hypertension and its prospects. Hypertens Res. 2019;42:301–5.
Sata F., Fukuoka H., Hanson M. (eds) Pre-emptive Medicine: Public Health Aspects of Developmental Origins of Health and Disease. Current Topics in Environmental Health and Preventive Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-13-2194-8_4.
World Health Organization. Global Nutrition Targets 2025: low birth weight policy brief. 2014. https://www.who.int/nutrition/publications/globaltargets2025_policybrief_lbw/en/. http://apps.who.int/iris/bitstream/10665/44844/1/9789241564441_eng.pdf.
Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes—a global concern. Nat Rev Nephrol. 2015;11:135–49.
Organization for Economic Coperation and Development. OECD Family Database. 2019. https://www.oecd.org/els/family/CO_1_3_Low_birth_weight.pdf.
Ohmi H, Hirooka K, Hata A, Mochizuki Y. Recent trend of increase in proportion of low birthweight infants in Japan. Int J Epidemiol. 2001;30:1269–71.
Ministry of Health, Labour and Welfare. Vital Statistics in Japan. 2019. http://www.mhlw.go.jp/english/database/db-hw/vs01.html. http://www.mhlw.go.jp/english/database/db-hw/dl/81-1a2en.pdf.
Lim J, Park HS. Trends in the prevalence of underweight, obesity, abdominal obesity and their related lifestyle factors in Korean young adults, 1998–2012. Obes Res Clin Pr. 2018;12:358–64.
Normile D. Staying slim during pregnancy carries a price. Science. 2018;361:440.
Kanda T, Takeda A, Hirose H, Abe T, Urai H, Inokuchi M, et al. Temporal trends in renal function and birthweight in Japanese adolescent males (1998-2015). Nephrol Dial Transpl. 2018;33:304–10.
Ministry of Education, Cultutre, Sports, Science and Technology. Annual Report of School Health Statistics Research. 2019. https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00400002&tstat=000001011648&cycle=0&tclass1=000001020135&stat_infid=000007567248.).
Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–53.
Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci USA. 2010;107:16757–8.
Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81.
Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–62.
Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71(Suppl 5):1344s–52s.
Hanson M. The birth and future health of DOHaD. J Dev Orig Health Dis. 2015;6:434–7.
Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28:2838–44.
Khalsa DD, Beydoun HA, Carmody JB. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr Nephrol. 2016;31:1509–16.
Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) Study. Am J Kidney Dis. 2008;51:10–20.
White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–61.
Das SK, Mannan M, Faruque AS, Ahmed T, McIntyre HD, Al Mamun A. Effect of birth weight on adulthood renal function: a bias-adjusted meta-analytic approach. Nephrology. 2016;21:547–65.
Silverwood RJ, Pierce M, Hardy R, Sattar N, Whincup P, Ferro C, et al. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 2013;84:1262–70.
Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346.
Ikezumi Y, Suzuki T, Karasawa T, Yamada T, Hasegawa H, Nishimura H, et al. Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am J Nephrol. 2013;38:149–57.
Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:71–6.
Rossing P, Tarnow L, Nielsen FS, Hansen BV, Brenner BM, Parving H-H. Low birth weight: a risk factor for development of diabetic nephropathy? Diabetes. 1995;44:1405–7.
Koike K, Ikezumi Y, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, et al. Glomerular density and volume in renal biopsy specimens of children with proteinuria relative to preterm birth and gestational age. Clin J Am Soc Nephrol. 2017;12:585–90.
Nelson RG, Morgenstern H, Bennett PH. Birth weight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol. 1998;148:650–6.
Knop Marianne R, Geng TT, Gorny Alexander W, Ding R, Li C, Ley Sylvia H, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta‐analysis of 7,646,267 participants from 135 studies. J Am Heart Assoc. 2018;7:e008870.
Levine RS, Hennekens CH, Jesse MJ. Blood pressure in prospective population based cohort of newborn and infant twins. BMJ. 1994;308:298–302.
Cruickshank J, Mzayek F, Liu L, Kieltyka L, Sherwin R, Webber L, et al. Origins of the “black/white” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation. 2005;111:1932–7.
de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–34.
Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–31.
Murai-Takeda A, Kanda T, Azegami T, Hirose H, Inokuchi M, Tokuyama H, et al. Low birth weight is associated with decline in renal function in Japanese male and female adolescents. Clin Exp Nephrol. 2019. https://doi.org/10.1007/s10157-019-01784-9.
Kawabe H, Shibata H, Hirose H, Tsujioka M, Saito I, Saruta T. Sexual differences in relationships between birth weight or current body weight and blood pressure or cholesterol in young Japanese students. Hypertens Res. 1999;22:169–72.
Mori M, Mori H, Yamori Y, Tsuda K. Low birth weight as cardiometabolic risk in Japanese high school girls. J Am Coll Nutr. 2012;31:39–44.
Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang-Karlsson S, Makitie O, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–63.
Hovi P, Andersson S, Räikkönen K, Strang-Karlsson S, Järvenpää A-L, Eriksson JG, et al. Ambulatory blood pressure in young adults with very low birth weight. J Pediatrics. 2010;156:54–9. e51
Kawabe H, Azegami T, Takeda A, Kanda T, Saito I, Saruta T, et al. Features of and preventive measures against hypertension in the young. Hypertens Res. 2019;42:935–48.
Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hote JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(Suppl 2):115–20.
Kanzaki G, Tsuboi N, Haruhara K, Koike K, Ogura M, Shimizu A, et al. Factors associated with a vicious cycle involving a low nephron number, hypertension and chronic kidney disease. Hypertens Res. 2015;38:633–41.
Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376(24):2349–57.
Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–47.
Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int. 2005;68:S68–77.
Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–84.
Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–3.
Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–74.
Hoy WE, Hughson MD, Singh G, Douglas-Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104–10.
Kanzaki G, Puelles VG, Cullen-McEwen LA, Hoy WE, Okabayashi Y, Tsuboi N, et al. New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI Insight. 2017;2:e94334. https://doi.org/10.1172/jci.insight.94334.
de Boer MP, Ijzerman RG, de Jongh RT, Eringa EC, Stehouwer CD, Smulders YM, et al. Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension. 2008;51:928–32.
Kistner A, Jacobson L, Jacobson SH, Svensson E, Hellstrom A. Low gestational age associated with abnormal retinal vascularization and increased blood pressure in adult women. Pediatr Res. 2002;51:675–80.
Mitchell P, Liew G, Rochtchina E, Wang JJ, Robaei D, Cheung N, et al. Evidence of arteriolar narrowing in low-birth-weight children. Circulation. 2008;118:518–24.
Martyn C, Greenwald S. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–55.
Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–94.
Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, et al. Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res. 1998;40:600–6.
Asada N, Tsukahara T, Furuhata M, Matsuoka D, Noda S, Naganuma K, et al. Polycythemia, capillary rarefaction, and focal glomerulosclerosis in two adolescents born extremely low birth weight and premature. Pediatr Nephrol. 2017;32:1275–8.
Abdulmahdi W, Rabadi MM, Jules E, Marghani Y, Marji N, Leung J, et al. Kidney dysfunction in the low-birth weight murine adult: implications of oxidative stress. Am J Physiol-Ren Physiol. 2018;315:F583–94.
Tennant IA, Barnett AT, Thompson DS, Kips J, Boyne MS, Chung EE, et al. Impaired cardiovascular structure and function in adult survivors of severe acute malnutrition. Hypertension. 2014;64:664–71.
Lane RH. Fetal programming, epigenetics, and adult onset disease. Clin Perinatol. 2014;41:815–31.
Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–9.
Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014;5:5592.
Rangel M, dos Santos JC, Ortiz PH, Hirata M, Jasiulionis MG, Araujo RC, et al. Modification of epigenetic patterns in low birth weight children: importance of hypomethylation of the ACE gene promoter. PLoS ONE. 2014;9:e106138.
Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS ONE. 2010;5:e9237.
El-Dahr SS. DNA methylation links intrauterine stress with abnormal nephrogenesis. Nat Rev Nephrol. 2019;15:196.
Wanner N, Vornweg J, Combes A, Wilson S, Plappert J, Rafflenbeul G, et al. DNA methyltransferase 1 controls nephron progenitor cell renewal and differentiation. J Am Soc Nephrol. 2019;30:63–78.
IJzerman RG, Stehouwer CD, De Geus EJ, Van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation. 2003;108:566–71.
Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, et al. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension. 2013;61:828–34.
Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–48.
Coats LE, Davis GK, Newsome AD, Ojeda NB, Alexander BT. Low birth weight, blood pressure and renal susceptibility. Curr Hypertens Rep. 2019;21:62.
Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15:20.
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–5.
Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–81.
Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439.
Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–8.
Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–29.
Blush J, Lei J, Ju W, Silbiger S, Pullman J, Neugarten J. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int. 2004;66:2148–54.
Li S, Chen SC, Shlipak M, Bakris G, McCullough PA, Sowers J, et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 2008;73:637–42.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, et al. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–33.
Nagai M, Ohkubo T, Murakami Y, Takashima N, Kadota A, Miyagawa N, et al. Secular trends of the impact of overweight and obesity on hypertension in Japan, 1980–2010. Hypertens Res. 2015;38:790.
Gluckman PD, Seng CY, Fukuoka H, Beedle AS, Hanson MA. Low birthweight and subsequent obesity in Japan. Lancet. 2007;369:1081–2.
Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–63.
Klebanoff MA, Secher NJ, Mednick BR, Schulsinger C. Maternal size at birth and the development of hypertension during pregnancy: a test of the Barker hypothesis. Arch Intern Med. 1999;159:1607–12.
Innes KE, Marshall JA, Byers TE, Calonge N. A woman’s own birth weight and gestational age predict her later risk of developing preeclampsia, a precursor of chronic disease. Epidemiology. 1999;10:153–60.
Vikse BE. Pre-eclampsia and the risk of kidney disease. Lancet. 2013;382:104–6.
Gallo LA, Tran M, Master JS, Moritz KM, Wlodek ME. Maternal adaptations and inheritance in the transgenerational programming of adult disease. Cell Tissue Res. 2012;349:863–80.
Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–8.
Briffa JF, Wlodek ME, Moritz KM. Transgenerational programming of nephron deficits and hypertension. Paper presented at: Seminars in cell & developmental biology 2018.
Qian M, Chou S-Y, Gimenez L, Liu J-T. The intergenerational transmission of low birth weight and intrauterine growth restriction: a large cross-generational cohort study in Taiwan. Matern Child Health J. 2017;21:1512–21.
Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–6.
Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–20.
Kazmi N, Sharp GC, Reese SE, Vehmeijer FO, Lahti J, Page CM, et al. Hypertensive disorders of pregnancy and DNA methylation in newborns. Hypertension. 2019;74:375–83.
Luyckx VA, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet. 2017;390:424–8.
Crump C. Medical history taking in adults should include questions about preterm birth. BMJ. 2014;349:g4860.
Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–43.
Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–30.
Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–31.
Lurbe E, Garcia-Vicent C, Torro MI, Aguilar F, Redon J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at 5 years of age. Hypertension. 2014;63:1326–32.
Intapad S, Dasinger JH, Johnson JM, Brown AD, Ojeda NB, Alexander BT. Male and female intrauterine growth-restricted offspring differ in blood pressure, renal function, and glucose homeostasis responses to a postnatal diet high in fat and sugar. Hypertension. 2019;73:620–9.
Luyckx VA, Compston CA, Simmen T, Mueller TF. Accelerated senescence in kidneys of low-birth-weight rats after catch-up growth. Am J Physiol-Ren Physiol. 2009;297:F1697–705.
Jones DW, Clark D 3rd, Hall ME. Preterm birth is associated with increased blood pressure in young adults: important opportunities for blood pressure management. J Am Heart Assoc. 2019;8:e013109.
Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–80.
Zhang WB, Pincus Z. Predicting all-cause mortality from basic physiology in the Framingham Heart Study. Aging Cell. 2016;15:39–48.
Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol-Ren Physiol. 2004;287:F262–7.
Hibino S, Abe Y, Watanabe S, Yamaguchi Y, Nakano Y, Tatsuno M, et al. Proteinuria caused by glomerular hypertension during adolescence associated with extremely premature birth: a report of two cases. Pediatr Nephrol. 2015;30:1889–92.
Orskov B, Christensen KB, Feldt-Rasmussen B, Strandgaard S. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney Int. 2012;81:919–24.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanda, T., Murai-Takeda, A., Kawabe, H. et al. Low birth weight trends: possible impacts on the prevalences of hypertension and chronic kidney disease. Hypertens Res 43, 859–868 (2020). https://doi.org/10.1038/s41440-020-0451-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0451-z
Keywords:
This article is cited by
-
Interactive and joint associations of prenatal ozone and PM2.5 exposure with fetal growth restriction: an Iranian nationwide birth cohort study
BMC Public Health (2025)
-
Subsequent high blood pressure and hypertension by hypertensive disorders of pregnancy: the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study
Hypertension Research (2025)
-
Hypertensive disorders of pregnancy: impact on future blood pressure and cardiovascular disease
Hypertension Research (2025)
-
Multi-omic insights of preeclampsia and cardiovascular health outcomes
Communications Medicine (2025)
-
Epigenetic aging mediates the association between life course socioeconomic status and decrements in kidney function across a decade
GeroScience (2025)