Abstract
Recent advances in mechanobiology and the discovery of mechanosensitive ion channels have opened a new era of research on hypertension and related diseases. Piezo1 and Piezo2, first reported in 2010, are regarded as bona fide mechanochannels that mediate various biological and pathophysiological phenomena in multiple tissues and organs. For example, Piezo channels have pivotal roles in blood pressure control, triggering shear stress-induced nitric oxide synthesis and vasodilation, regulating baroreflex in the carotid sinus and aorta, and releasing renin from renal juxtaglomerular cells. Herein, we provide an overview of recent literature on the roles of Piezo channels in the pathogenesis of hypertension and related kidney damage, including our experimental data on the involvement of Piezo1 in podocyte injury and that of Piezo2 in renin expression and renal fibrosis in animal models of hypertensive nephropathy.

The mechanosensitive ion channels Piezo1 and Piezo2 play various roles in the pathogenesis of systemic hypertension by acting on vascular endothelial cells, baroreceptors in the carotid artery and aorta, and the juxtaglomerular apparatus. Piezo channels also contribute to hypertensive nephropathy by acting on mesangial cells, podocytes, and perivascular mesenchymal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thompson DAW. On growth and form. Cambridge, UK: University Press; 1917.
Nagase T, Nagase M, Machida M, Yamagishi M. Hedgehog signaling: a biophysical or biomechanical modulator in embryonic development? Ann N Y Acad Sci. 2007;1101:412–38.
Davis MJ, Earley S, Li YS, Chien S. Vascular mechanotransduction. Physiol Rev. 2023;103:1247–421.
Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–79.
Kefauver JM, Ward AB, Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. 2020;587:567–76.
Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling (Nobel Lecture). Angew Chem Int Ed Engl. 1999;38:1856–68.
Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide (Nobel Lecture). Angew Chem Int Ed Engl. 1999;38:1870–80.
Ignarro LJ. Nitric oxide: a unique endogenous signaling molecule in vascular biology (Nobel Lecture). Angew Chem Int Ed Engl. 1999;38:1882–92.
Heymans C, Neil E. Reflexogenic areas of the cardiovascular system. London, UK: Churchill, Ltd; 1958.
Tobian L, Tomboulian A, Janecek J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959;38:605–10.
Skinner SL, McCubbin JW, Page IH. Renal baroreceptor control of renin secretion. Science. 1963;141:814–6.
Quillon A, Fromy B, Debret R. Endothelium microenvironment sensing leading to nitric oxide mediated vasodilation: a review of nervous and biomechanical signals. Nitric Oxide. 2015;45:20–6.
Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–97.
McCleskey EW. A molecular sensor for the baroreceptor reflex? Neuron. 2009;64:776–7.
Seghers F, Yerna X, Zanou N, Devuyst O, Vennekens R, Nilius B, et al. TRPV4 participates in pressure-induced inhibition of renin secretion by juxtaglomerular cells. J Physiol. 2016;594:7327–40.
Dworkin LD, Hostetter TH, Rennke HG, Brenner BM. Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest. 1984;73:1448–61.
Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–9.
Kimura G, Brenner BM. The renal basis for salt sensitivity in hypertension. In: Laragh JH, Brenner BM, editors. Hypertension, pathophysiology, diagnosis, and management. Vol. 1. 2nd ed. New York, NY, USA: Raven Press; 1995. pp. 1569–88.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–93.
Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50:877–83.
Nagase M, Fujita T. Mineralocorticoid receptor activation in obesity hypertension. Hypertens Res. 2009;32:649–57.
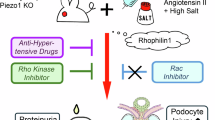
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43.
Yuan X, Zhao X, Wang W, Li C. Mechanosensing by Piezo1 and its implications in the kidney. Acta Physiol. 2024;240:e14152. https://doi.org/10.1111/apha.14152.
Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:2297–301.
Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–8.
Du Y, Xu B, Li Q, Peng C, Yang K. The role of mechanically sensitive ion channel Piezo1 in bone remodeling. Front Bioeng Biotechnol. 2024;12:1342149. https://doi.org/10.3389/fbioe.2024.1342149.
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60.
Fang XZ, Zhou T, Xu JQ, Wang YX, Sun MM, He YJ, et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021;11:13. https://doi.org/10.1186/s13578-020-00522-z.
Lai A, Cox CD, Chandra Sekar N, Thurgood P, Jaworowski A, Peter K, et al. Mechanosensing by Piezo1 and its implications for physiology and various pathologies. Biol Rev Camb Philos Soc. 2022;97:604–14.
Szczot M, Nickolls AR, Lam RM, Chesler AT. The form and function of PIEZO2. Annu Rev Biochem. 2021;90:507–34.
Earley S, Santana LF, Lederer WJ. The physiological sensor channels TRP and piezo: Nobel Prize in Physiology or Medicine 2021. Physiol Rev. 2022;102:1153–8.
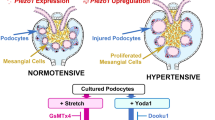
Ogino S, Yoshikawa K, Nagase T, Mikami K, Nagase M. Roles of the mechanosensitive ion channel Piezo1 in the renal podocyte injury of experimental hypertensive nephropathy. Hypertens Res. 2024;47:747–59.
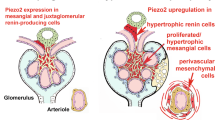
Mochida Y, Ochiai K, Nagase T, Nonomura K, Akimoto Y, Fukuhara H, et al. Piezo2 expression and its alteration by mechanical forces in mouse mesangial cells and renin-producing cells. Sci Rep. 2022;12:4197. https://doi.org/10.1038/s41598-022-07987-7.
Ochiai K, Mochida Y, Nagase T, Fukuhara H, Yamaguchi Y, Nagase M. Upregulation of Piezo2 in the mesangial, renin, and perivascular mesenchymal cells of the kidney of Dahl salt-sensitive hypertensive rats and its reversal by esaxerenone. Hypertens Res. 2023;46:1234–46.
Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527:64–9.
Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017;6:e33660. https://doi.org/10.7554/eLife.33660.
Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554:481–6.
Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487–92.
Yang X, Lin C, Chen X, Li S, Li X, Xiao B. Structure deformation and curvature sensing of PIEZO1 in lipid membranes. Nature. 2022;604:377–83.
Zhao Q, Zhou H, Li X, Xiao B. The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019;286:2461–70.
Jiang Y, Yang X, Jiang J, Xiao B. Structural designs and mechanogating mechanisms of the mechanosensitive Piezo channels. Trends Biochem Sci. 2021;46:472–88.
Xu X, Liu S, Liu H, Ru K, Jia Y, Wu Z, et al. Piezo channels: awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int J Mol Sci. 2021;22:6429. https://doi.org/10.3390/ijms22126429.
Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q, et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun. 2018;9:1300. https://doi.org/10.1038/s41467-018-03570-9.
Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019;573:230–4.
Mulhall EM, Gharpure A, Lee RM, Dubin AE, Aaron JS, Marshall KL, et al. Direct observation of the conformational states of PIEZO1. Nature. 2023;620:1117–25.
Wang L, Zhou H, Zhang M, Liu W, Deng T, Zhao Q, et al. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature. 2019;573:225–9.
Taberner FJ, Prato V, Schaefer I, Schrenk-Siemens K, Heppenstall PA, Lechner SG. Structure-guided examination of the mechanogating mechanism of PIEZO2. Proc Natl Acad Sci USA. 2019;116:14260–9.
Qin L, He T, Chen S, Yang D, Yi W, Cao H, et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021;9:44. https://doi.org/10.1038/s41413-021-00168-8.
Coste B, Delmas P. PIEZO ion channels in cardiovascular functions and diseases. Circ Res. 2024;134:572–91.
Zhou Z, Martinac B. Mechanisms of PIEZO channel inactivation. Int J Mol Sci. 2023;24:14113. https://doi.org/10.3390/ijms241814113.
Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–81.
Huang J, Zhang K, Du R, Liu W, Zhang H, Tian T, et al. The Janus-faced role of Piezo1 in cardiovascular health under mechanical stimulation. Genes Dis. 2023;10:1956–68.
Vasileva V, Chubinskiy-Nadezhdin V. Regulation of PIEZO1 channels by lipids and the structural components of extracellular matrix/cell cytoskeleton. J Cell Physiol. 2023;238:918–30.
Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF, et al. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun. 2019;10:1200. https://doi.org/10.1038/s41467-019-09055-7.
Ma S, Dubin AE, Romero LO, Loud M, Salazar A, Chu S, et al. Excessive mechanotransduction in sensory neurons causes joint contractures. Science. 2023;379:201–6.
Shi J, Hyman AJ, De Vecchis D, Chong J, Lichtenstein L, Futers TS, et al. Sphingomyelinase disables inactivation in endogenous PIEZO1 channels. Cell Rep. 2020;33:108225. https://doi.org/10.1016/j.celrep.2020.108225.
Buyan A, Cox CD, Barnoud J, Li J, Chan HSM, Martinac B, et al. Piezo1 forms specific, functionally important interactions with phosphoinositides and cholesterol. Biophys J. 2020;119:1683–97.
Lin Y, Buyan A, Corry B. Characterizing the lipid fingerprint of the mechanosensitive channel Piezo2. J Gen Physiol. 2022;154:e202113064. https://doi.org/10.1085/jgp.202113064.
Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M, et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 2013;14:1143–8.
Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun. 2017;8:1797. https://doi.org/10.1038/s41467-017-01712-z.
Wetzel C, Pifferi S, Picci C, Gök C, Hoffmann D, Bali KK, et al. Small-molecule inhibition of STOML3 oligomerization reverses pathological mechanical hypersensitivity. Nat Neurosci. 2017;20:209–18.
Wang H, Yuan Z, Wang B, Li B, Lv H, He J, et al. COMP (Cartilage Oligomeric Matrix Protein), a novel PIEZO1 regulator that controls blood pressure. Hypertension. 2022;79:549–61.
Zhou Z, Ma X, Lin Y, Cheng D, Bavi N, Secker GA, et al. MyoD-family inhibitor proteins act as auxiliary subunits of Piezo channels. Science. 2023;381:799–804.
Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry. 2011;50:6295–300.
Suchyna TM, Tape SE, Koeppe RE 2nd, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–40.
Suchyna TM. Piezo channels and GsMTx4: two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog Biophys Mol Biol. 2017;130:244–53.
Alcaino C, Knutson K, Gottlieb PA, Farrugia G, Beyder A. Mechanosensitive ion channel Piezo2 is inhibited by D-GsMTx4. Channels. 2017;11:245–53.
Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon. 2007;49:249–70.
Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015;4:e07369. https://doi.org/10.7554/eLife.07369.
Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ, et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharm. 2018;175:1744–59.
Parsonage G, Cuthbertson K, Endesh N, Murciano N, Hyman AJ, Revill CH, et al. Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1. Br J Pharm. 2023;180:2039–63.
Jiang W, Wijerathne TD, Zhang H, Lin YC, Jo S, Im W, et al. Structural and thermodynamic framework for PIEZO1 modulation by small molecules. Proc Natl Acad Sci USA. 2023;120:e2310933120. https://doi.org/10.1073/pnas.2310933120.
Del Rosario JS, Gabrielle M, Yudin Y, Rohacs T. TMEM120A/TACAN inhibits mechanically activated PIEZO2 channels. J Gen Physiol. 2022;154:e202213164. https://doi.org/10.1085/jgp.202213164.
Gabrielle M, Yudin Y, Wang Y, Su X, Rohacs T. Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity. bioRxiv. 2024. https://doi.org/10.1101/2024.03.01.582964.
Shinge SAU, Zhang D, Achu Muluh T, Nie Y, Yu F. Mechanosensitive Piezo1 channel evoked-mechanical signals in atherosclerosis. J Inflamm Res. 2021;14:3621–36.
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA. 2014;111:10347–52.
Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–82.
Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126:4527–36.
Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest. 2019;129:2775–91.
Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8:350. https://doi.org/10.1038/s41467-017-00429-3.
Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, et al. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 2015;13:1161–71.
Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120:1908–15.
Jankovsky N, Caulier A, Demagny J, Guitton C, Djordjevic S, Lebon D, et al. Recent advances in the pathophysiology of PIEZO1-related hereditary xerocytosis. Am J Hematol. 2021;96:1017–26.
Zhang Y, Su SA, Li W, Ma Y, Shen J, Wang Y, et al. Piezo1-mediated mechanotransduction promotes cardiac hypertrophy by impairing calcium homeostasis to activate calpain/calcineurin signaling. Hypertension. 2021;78:647–60.
Lim GB. Piezo1 senses pressure overload and initiates cardiac hypertrophy. Nat Rev Cardiol. 2022;19:503. https://doi.org/10.1038/s41569-022-00746-1.
Nonomura K, Lukacs V, Sweet DT, Goddard LM, Kanie A, Whitwam T, et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci USA. 2018;115:12817–22.
Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573:69–74.
Atcha H, Jairaman A, Holt JR, Meli VS, Nagalla RR, Veerasubramanian PK, et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat Commun. 2021;12:3256. https://doi.org/10.1038/s41467-021-23482-5.
Xia K, Chen X, Wang W, Liu Q, Zhao M, Ma J, et al. Roles of mechanosensitive ion channels in immune cells. Heliyon. 2024;10:e23318. https://doi.org/10.1016/j.heliyon.2023.e23318.
Ma S, Dubin AE, Zhang Y, Mousavi SAR, Wang Y, Coombs AM, et al. A role of PIEZO1 in iron metabolism in mice and humans. Cell. 2021;184:969–82.e13.
Swain SM, Liddle RA. Mechanosensing Piezo channels in gastrointestinal disorders. J Clin Invest. 2023;133:e171955. https://doi.org/10.1172/jci171955.
Martins JR, Penton D, Peyronnet R, Arhatte M, Moro C, Picard N, et al. Piezo1-dependent regulation of urinary osmolarity. Pflug Arch. 2016;468:1197–206.
Fu Y, Wan P, Zhang J, Li X, Xing J, Zou Y, et al. Targeting mechanosensitive Piezo1 alleviated renal fibrosis through p38MAPK-YAP pathway. Front Cell Dev Biol. 2021;9:741060. https://doi.org/10.3389/fcell.2021.741060.
Zhao X, Kong Y, Liang B, Xu J, Lin Y, Zhou N, et al. Mechanosensitive Piezo1 channels mediate renal fibrosis. JCI Insight. 2022;7:e152330. https://doi.org/10.1172/jci.insight.152330.
He Y, Deng B, Liu S, Luo S, Ning Y, Pan X, et al. Myeloid Piezo1 deletion protects renal fibrosis by restraining macrophage infiltration and activation. Hypertension. 2022;79:918–31.
Drobnik M, Smólski J, Grądalski Ł, Niemirka S, Młynarska E, Rysz J, et al. Mechanosensitive cation channel Piezo1 is involved in renal fibrosis induction. Int J Mol Sci. 2024;25:1718. https://doi.org/10.3390/ijms25031718.
Zheng Q, Liu H, Yu W, Dong Y, Zhou L, Deng W, et al. Mechanical properties of the brain: focus on the essential role of Piezo1-mediated mechanotransduction in the CNS. Brain Behav. 2023;13:e3136. https://doi.org/10.1002/brb3.3136.
Qiu X, Deng Z, Wang M, Feng Y, Bi L, Li L. Piezo protein determines stem cell fate by transmitting mechanical signals. Hum Cell. 2023;36:540–53.
Otero-Sobrino Á, Blanco-Carlón P, Navarro-Aguadero M, Gallardo M, Martínez-López J, Velasco-Estévez M. Mechanosensitive ion channels: their physiological importance and potential key role in cancer. Int J Mol Sci. 2023;24:13710. https://doi.org/10.3390/ijms241813710.
Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–5.
Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–21.
Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–6.
Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci. 2015;18:1756–62.
Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2017;595:79–91.
Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, et al. PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature. 2020;588:290–5.
García-Mesa Y, Cárcaba L, Coronado C, Cobo R, Martín-Cruces J, García-Piqueras J, et al. Glans clitoris innervation: PIEZO2 and sexual mechanosensitivity. J Anat. 2021;238:446–54.
García-Mesa Y, García-Piqueras J, Cobo R, Martín-Cruces J, Suazo I, García-Suárez O, et al. Sensory innervation of the human male prepuce: Meissner’s corpuscles predominate. J Anat. 2021;239:892–902.
Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science. 2018;360:530–3.
Szczot M, Liljencrantz J, Ghitani N, Barik A, Lam R, Thompson JH, et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med. 2018;10:eaat9892. https://doi.org/10.1126/scitranslmed.aat9892.
Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kühnemund J, et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med. 2018;10:eaat9897. https://doi.org/10.1126/scitranslmed.aat9897.
Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464–7.
Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–81.
Madar J, Tiwari N, Smith C, Sharma D, Shen S, Elmahdi A, et al. Piezo2 regulates colonic mechanical sensitivity in a sex specific manner in mice. Nat Commun. 2023;14:2158. https://doi.org/10.1038/s41467-023-37683-7.
Lam RM, von Buchholtz LJ, Falgairolle M, Osborne J, Frangos E, Servin-Vences MR, et al. PIEZO2 and perineal mechanosensation are essential for sexual function. Science. 2023;381:906–10.
Delmas P, Parpaite T, Coste B. PIEZO channels and newcomers in the mammalian mechanosensitive ion channel family. Neuron. 2022;110:2713–27.
Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun. 2015;6:8329. https://doi.org/10.1038/ncomms9329.
Amado NG, Nosyreva ED, Thompson D, Egeland TJ, Ogujiofor OW, Yang M, et al. PIEZO1 loss-of-function compound heterozygous mutations in the rare congenital human disorder Prune Belly Syndrome. Nat Commun. 2024;15:339. https://doi.org/10.1038/s41467-023-44594-0.
Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc Natl Acad Sci USA. 2013;110:4667–72.
McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HI, et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet. 2014;94:734–44.
Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, et al. The Role of PIEZO2 in Human Mechanosensation. N Engl J Med. 2016;375:1355–64.
Lim XR, Harraz OF. Mechanosensing by vascular endothelium. Annu Rev Physiol. 2024;86:71–97.
Beech DJ, Kalli AC. Force sensing by Piezo channels in cardiovascular health and disease. Arterioscler Thromb Vasc Biol. 2019;39:2228–39. https://doi.org/10.1161/atvbaha.119.313348.
Douguet D, Patel A, Xu A, Vanhoutte PM, Honoré E. Piezo ion channels in cardiovascular mechanobiology. Trends Pharm Sci. 2019;40:956–70.
Albarrán-Juárez J, Iring A, Wang S, Joseph S, Grimm M, Strilic B, et al. Piezo1 and G(q)/G(11) promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215:2655–72.
Yang H, Tenorio Lopes L, Barioni NO, Roeske J, Incognito AV, Baker J, et al. The molecular makeup of peripheral and central baroreceptors: stretching a role for Transient Receptor Potential (TRP), Epithelial Sodium Channel (ENaC), Acid Sensing Ion Channel (ASIC), and Piezo channels. Cardiovasc Res. 2022;118:3052–70.
Cui CP, Xiong X, Zhao JX, Fu DH, Zhang Y, Ma PB, et al. Piezo1 channel activation facilitates baroreflex afferent neurotransmission with subsequent blood pressure reduction in control and hypertension rats. Acta Pharm Sin. 2024;45:76–86.
Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron. 1998;21:1435–41.
Lau OC, Shen B, Wong CO, Tjong YW, Lo CY, Wang HC, et al. TRPC5 channels participate in pressure-sensing in aortic baroreceptors. Nat Commun. 2016;7:11947. https://doi.org/10.1038/ncomms11947.
Lau OC, Shen B, Wong CO, Tjong YW, Lo CY, Wang HC, et al. Author Correction: TRPC5 channels participate in pressure-sensing in aortic baroreceptors. Nat Commun. 2018;9:16184. https://doi.org/10.1038/ncomms16184.
Min S, Chang RB, Prescott SL, Beeler B, Joshi NR, Strochlic DE, et al. Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Rep. 2019;29:2192–201.e3.
Huo L, Gao Y, Zhang D, Wang S, Han Y, Men H, et al. Piezo2 channel in nodose ganglia neurons is essential in controlling hypertension in a pathway regulated directly by Nedd4-2. Pharm Res. 2021;164:105391. https://doi.org/10.1016/j.phrs.2020.105391.
Stocker SD, Sved AF, Andresen MC. Missing pieces of the Piezo1/Piezo2 baroreceptor hypothesis: an autonomic perspective. J Neurophysiol. 2019;122:1207–12.
Watanabe H, Belyea BC, Paxton RL, Li M, Dzamba BJ, DeSimone DW, et al. Renin cell baroreceptor, a nuclear mechanotransducer central for homeostasis. Circ Res. 2021;129:262–76.
Yamaguchi H, Gomez RA, Sequeira-Lopez MLS. Renin cells, from vascular development to blood pressure sensing. Hypertension. 2023;80:1580–9.
Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9.
Dalghi MG, Clayton DR, Ruiz WG, Al-Bataineh MM, Satlin LM, Kleyman TR, et al. Expression and distribution of PIEZO1 in the mouse urinary tract. Am J Physiol Ren Physiol. 2019;317:F303–21.
Lu Y, Ye Y, Yang Q, Shi S. Single-cell RNA-sequence analysis of mouse glomerular mesangial cells uncovers mesangial cell essential genes. Kidney Int. 2017;92:504–13.
Karaiskos N, Rahmatollahi M, Boltengagen A, Liu H, Hoehne M, Rinschen M, et al. A single-cell transcriptome atlas of the mouse glomerulus. J Am Soc Nephrol. 2018;29:2060–8.
He B, Chen P, Zambrano S, Dabaghie D, Hu Y, Möller-Hackbarth K, et al. Single-cell RNA sequencing reveals the mesangial identity and species diversity of glomerular cell transcriptomes. Nat Commun. 2021;12:2141. https://doi.org/10.1038/s41467-021-22331-9.
Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Rep. 2014;3:650–62.
Sequeira-Lopez ML, Lin EE, Li M, Hu Y, Sigmund CD, Gomez RA. The earliest metanephric arteriolar progenitors and their role in kidney vascular development. Am J Physiol Regul Integr Comp Physiol. 2015;308:R138–49.
Sequeira-Lopez MLS, Gomez RA. Renin cells, the kidney, and hypertension. Circ Res. 2021;128:887–907.
Yang X, Zeng H, Wang L, Luo S, Zhou Y. Activation of Piezo1 downregulates renin in juxtaglomerular cells and contributes to blood pressure homeostasis. Cell Biosci. 2022;12:197. https://doi.org/10.1186/s13578-022-00931-2.
Hill RZ, Shirvan S, Burquez S, Dubin AE, Servin-Vences MR, Miner JH, et al. Renal mechanotransduction is an essential regulator of renin. bioRxiv. 2023. https://doi.org/10.1101/2023.11.04.565646.
Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–64.
Endlich K, Kliewe F, Endlich N. Stressed podocytes-mechanical forces, sensors, signaling and response. Pflug Arch. 2017;469:937–49.
Haydak J, Azeloglu EU. Role of biophysics and mechanobiology in podocyte physiology. Nat Rev Nephrol. 2024;20:371–85.
Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079–86.
Forst AL, Olteanu VS, Mollet G, Wlodkowski T, Schaefer F, Dietrich A, et al. Podocyte purinergic P2X4 channels are mechanotransducers that mediate cytoskeletal disorganization. J Am Soc Nephrol. 2016;27:848–62.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Chambers L, Dorrance AM. Regulation of ion channels in the microcirculation by mineralocorticoid receptor activation. Curr Top Membr. 2020;85:151–85.
Black LM, Lever JM, Agarwal A. Renal inflammation and fibrosis: a double-edged sword. J Histochem Cytochem. 2019;67:663–81.
Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–80.
Shaw I, Rider S, Mullins J, Hughes J, Péault B. Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol. 2018;14:521–34.
Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26.
Arai H, Yanagita M. Janus-faced: molecular mechanisms and versatile nature of renal fibrosis. Kidney360. 2020;1:697–704.
Kuppe C, Ibrahim MM, Kranz J, Zhang X, Ziegler S, Perales-Patón J, et al. Decoding myofibroblast origins in human kidney fibrosis. Nature. 2021;589:281–6.
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97.
Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, et al. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–90.
Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66.
Minatoguchi S, Saito S, Furuhashi K, Sawa Y, Okazaki M, Shimamura Y, et al. A novel renal perivascular mesenchymal cell subset gives rise to fibroblasts distinct from classic myofibroblasts. Sci Rep. 2022;12:5389. https://doi.org/10.1038/s41598-022-09331-5.
Tanaka S, Portilla D, Okusa MD. Role of perivascular cells in kidney homeostasis, inflammation, repair and fibrosis. Nat Rev Nephrol. 2023;19:721–32.
Drury ER, Wu J, Gigliotti JC, Le TH. Sex differences in blood pressure regulation and hypertension: renal, hemodynamic, and hormonal mechanisms. Physiol Rev. 2024;104:199–251.
Asunción-Alvarez D, Palacios J, Ybañez-Julca RO, Rodriguez-Silva CN, Nwokocha C, Cifuentes F, et al. Calcium signaling in endothelial and vascular smooth muscle cells: sex differences and the influence of estrogens and androgens. Am J Physiol Heart Circ Physiol. 2024;326:H950–70.
John L, Ko NL, Gokin A, Gokina N, Mandalà M, Osol G. The Piezo1 cation channel mediates uterine artery shear stress mechanotransduction and vasodilation during rat pregnancy. Am J Physiol Heart Circ Physiol. 2018;315:H1019–26.
Arishe OO, McKenzie J, Dela Justina V, Dos Anjos Moraes R, Webb RC, Priviero F. Piezo1 channels mediate vasorelaxation of uterine arteries from pseudopregnant rats. Front Physiol. 2023;14:1140989. https://doi.org/10.3389/fphys.2023.1140989.
Barnett SD, Asif H, Buxton ILO. Novel identification and modulation of the mechanosensitive Piezo1 channel in human myometrium. J Physiol. 2023;601:1675–90.
Arishe OO, Ebeigbe AB, Webb RC. Mechanotransduction and uterine blood flow in preeclampsia: the role of mechanosensing Piezo 1 ion channels. Am J Hypertens. 2020;33:1–9.
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42.
Sehnal D, Bittrich S, Deshpande M, Svobodová R, Berka K, Bazgier V, et al. Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021;49:W431–7.
Acknowledgements
We thank Dr. Yuki Mochida, Dr. Koji Ochiai, Dr. Satoyuki Ogino, Dr. Kei Yoshikawa, and Dr. Kaori Mikami for their contributions to the experiments described in this review. We also thank Professor Emeritus George Matsumura for the illustration of Fig. 2 and Editage (www.editage.jp) for English language editing.
Funding
This work was supported in part by JSPS KAKENHI Grant Numbers JP17K09736, JP20K08616, JP23K07704, by Japan Agency for Medical Research and Development (18gm5810019h9903), and by the Salt Science Research Foundation, No. 2329.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagase, T., Nagase, M. Piezo ion channels: long-sought-after mechanosensors mediating hypertension and hypertensive nephropathy. Hypertens Res 47, 2786–2799 (2024). https://doi.org/10.1038/s41440-024-01820-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-024-01820-6
Keywords
This article is cited by
-
Podocyte-specific deletion of mechanochannel Piezo1 exacerbates proteinuria and podocyte injury in mouse hypertensive nephropathy
Hypertension Research (2026)
-
Recent advances and emerging perspectives in vascular and cardiovascular research: A 2025 update
Hypertension Research (2026)
-
Identification of putative baroreceptors in human aortic arch by histological and omics analyses
Hypertension Research (2025)
-
Upregulation of Piezo2 and increased extracellular matrix protein in diabetic kidney disease mice
Hypertension Research (2025)