Fig. 1
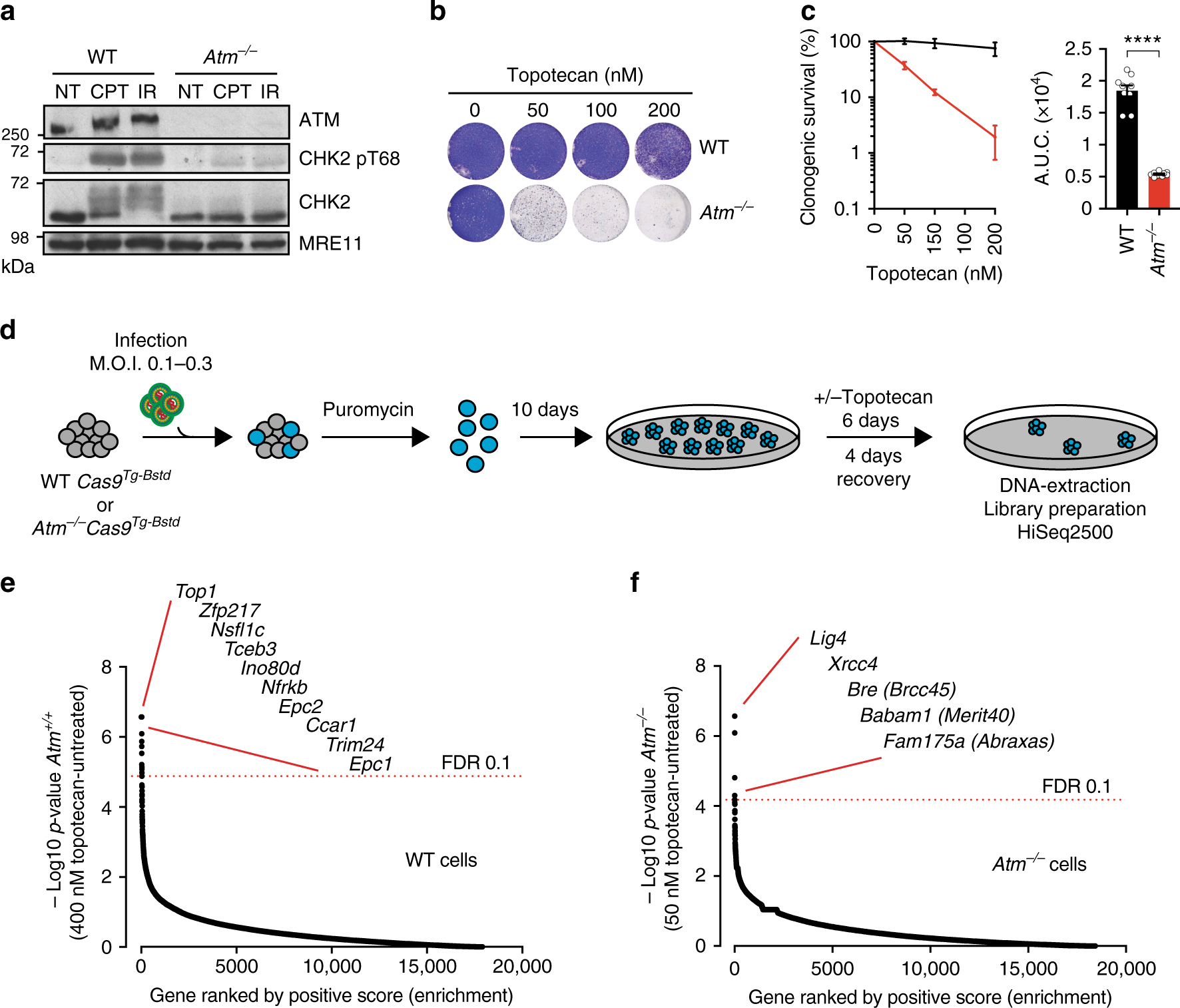
CRISPR-Cas9 screening in WT and ATM-deficient mESCs. a Representative immunoblot images show the absence of ATM protein and defective signaling through phosphorylation of its substrate CHK2 on Thr-68. NT untreated, CPT camptothecin (1 μM, 1 h), IR ionizing radiation (10 Gy, 1 h). b, c Crystal violet cell viability assay (b) and clonogenic survival assays (c) showing hypersensitivity of ATM-deficient cells to topotecan; n = 9/genotype; error bars s.e.m.; t = 15.17; df = 4; ****p < 0.0001; two-tailed Student’s t test based on AUC (area under the curve). d Outline of the CRISPR screen. ATM wild-type or ATM-deficient cells stably expressing Cas9 nuclease were infected with lentiviral particles containing the whole-genome sgRNA library, subjected to puromycin selection, and passaged to ensure loss of affected protein products. Puromycin-resistant Atm+/+ or Atm−/− cells were exposed, respectively, to 400 and 50 nM topotecan for 6 days, and resistant pools isolated. Genomic DNA was extracted from these and from parallel cell cultures treated in the absence of topotecan, and DNA libraries were prepared and sequenced using HiSeq2500. MOI multiplicity of infection. e, f Classification of the most enriched CRISPR-targeted genes in topotecan-resistant wild-type (WT) (e) and Atm−/− (f) mESCs. Dotted red lines represent positive enrichment false discovery rate (FDR) thresholds. Represented are the names of top hits with highest enrichment scores. The two other specific components of the BRCA1-A complex ranked 201/18,424 (Brcc3/Brcc36) and 1200/18,424 (Uimc1/Rap80). All data were analyzed by using MAGeCK and are available in Supplementary Data files 1 and 2. Images are representative of three individual experiments. Panels containing clonogenic survival assays (left) and AUC (right) were generated using GraphPad Prism 7. Bars represent mean ± s.e.m.; ****p < 0.0001; NS = not significant (p > 0.05); two-tailed Student’s t test following F test to confirm equal variance; df = 4. For each clonogenic experiment data is pooled from n = 3 individual experiments. Supporting data, including selection of Cas9 clones, crystal violet cell viability assays, library coverage plots and dropout as well as pathway enrichment analysis for both WT and Atm−/− cells are presented in Supplementary Figures 1 and 2 and Supplementary Data 3 and 4. Source data are provided as a Source Data file
