Fig. 1: Architecture of the Aβ(1-42) tetramer.
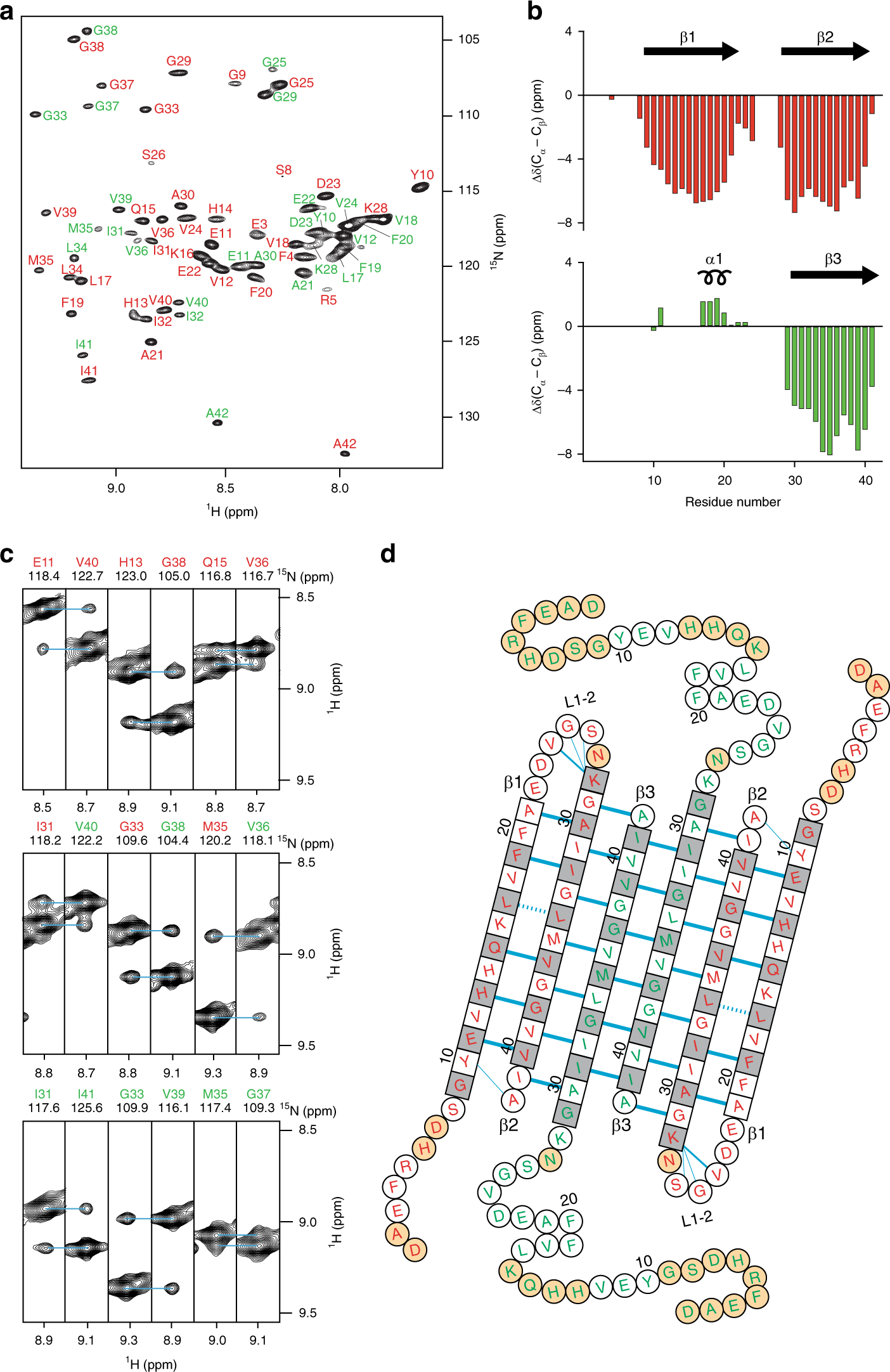
a Amide resonance assignments of the Aβ(1-42) tetramer. Two Aβ(1-42) subunits are detected and residues belonging to each of them are labeled in either red or green. b Three-bond averaged secondary chemical shifts versus residue number for the red (top) and the green (bottom) Aβ(1-42) subunits. Secondary structural elements derived from chemical shift indices are shown at the top with its corresponding number. Arrows indicate β-strands and helical symbols helices. c Strips from a 3D NH-NH NOESY spectrum defining long-range intra-monomer interactions between the red Aβ(1-42) subunit, long-range inter-monomer interactions between the red and the green Aβ(1-42) subunits, and long-range inter-dimer interactions between the two green Aβ(1-42) subunits. d The amino acid sequence of the Aβ(1-42) tetramer is arranged on the basis of the secondary and tertiary structure. Amino acids in square denote β-sheet secondary structure as identified by secondary chemical shifts; all other amino acids are in circles. Blue lines denote experimentally observed NOE contacts between two amide protons. Bold lines indicate strong NOEs typically observed between hydrogen-bonded residues in β-sheets. Dashed lines show probable contacts between protons with degenerate 1H chemical shifts. The side chains of white and gray residues point towards distinct sides of the β-sheet plane, respectively. Orange circles correspond to residues that could not be assigned. Sample conditions were 1 mM 2H,15N,13C Aβ(1-42) in 10 mM Tris, 28.5 mM DPC at pH 9.0 after incubation for 24 h at 37 °C. Source data are provided as a Source data file.
