Fig. 1: The CO32– problem.
From: The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem
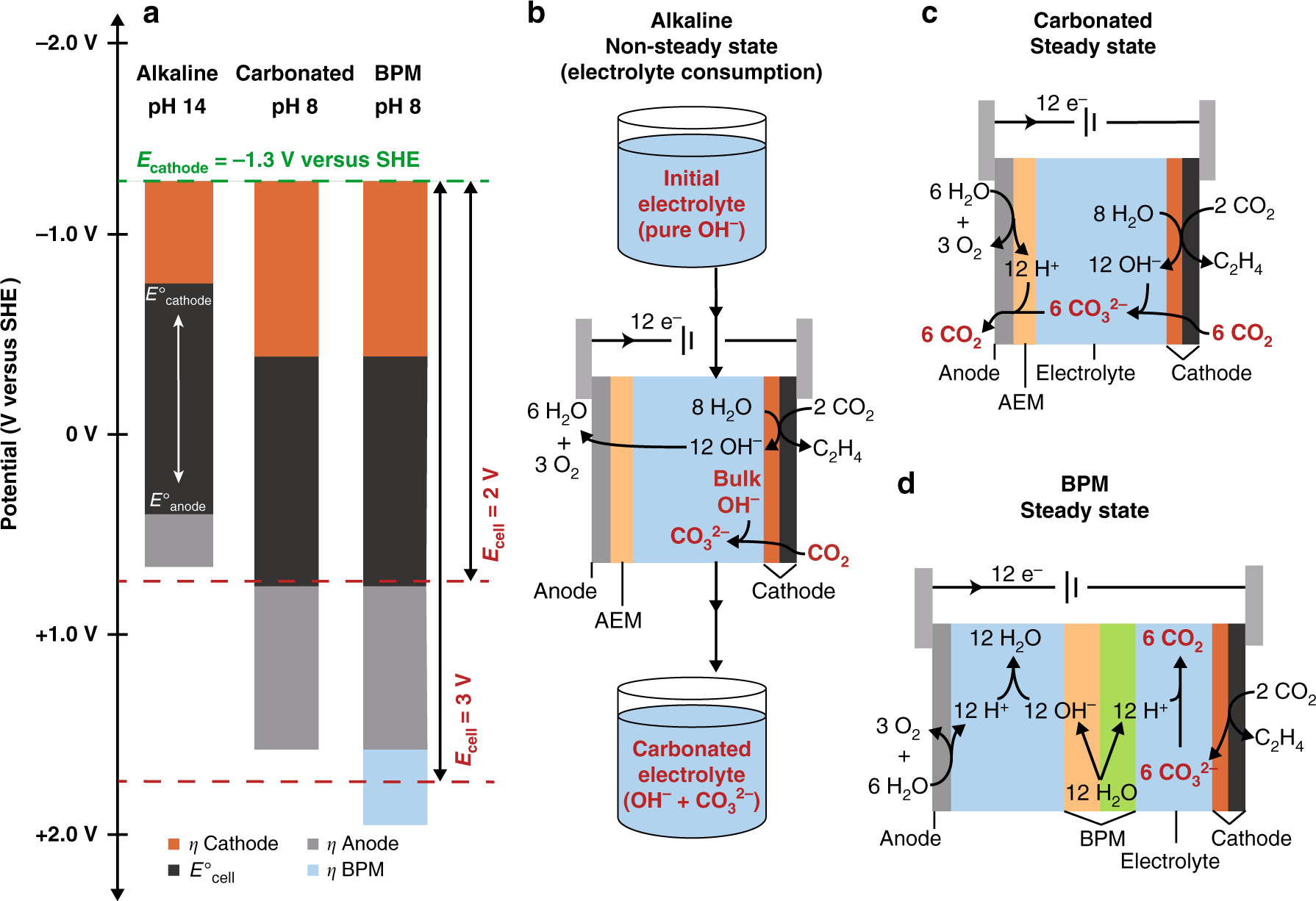
a Visualization of various contributors to the cell voltage for a CO2 electrolysis cell operating under alkaline conditions, carbonated conditions, or with a bipolar membrane (BPM). The contributions shown are the thermodynamic cell potential (E°cell), and cathode, anode, and BPM overpotentials (η). Electrode potentials are referenced to the SHE scale. For simplicity, cell resistance was not included, which would add to the cell voltage. The green dotted line represents the least negative cathode potential reported for low-temperature CO2 reduction at >200 mA cm–2 7. The red dotted lines provide a visual reference for cell voltages. The thermodynamic potentials for the cathode (E°cathode) and anode (E°anode) shift positive versus SHE as the pH is decreased. Alkaline conditions minimize cell voltage but cannot be maintained at steady state because of CO32– formation. b Schematic of anion transport in an alkaline flow cell showing OH– consumption by CO2. c Schematic of a cell at steady state after carbonation showing the carbon loss due to CO2 released at the anode. d Schematic of a BPM cell at steady state.
