Fig. 1: N-Activation strategies for aziridination.
From: N-Aminopyridinium reagents as traceless activating groups in the synthesis of N-Aryl aziridines
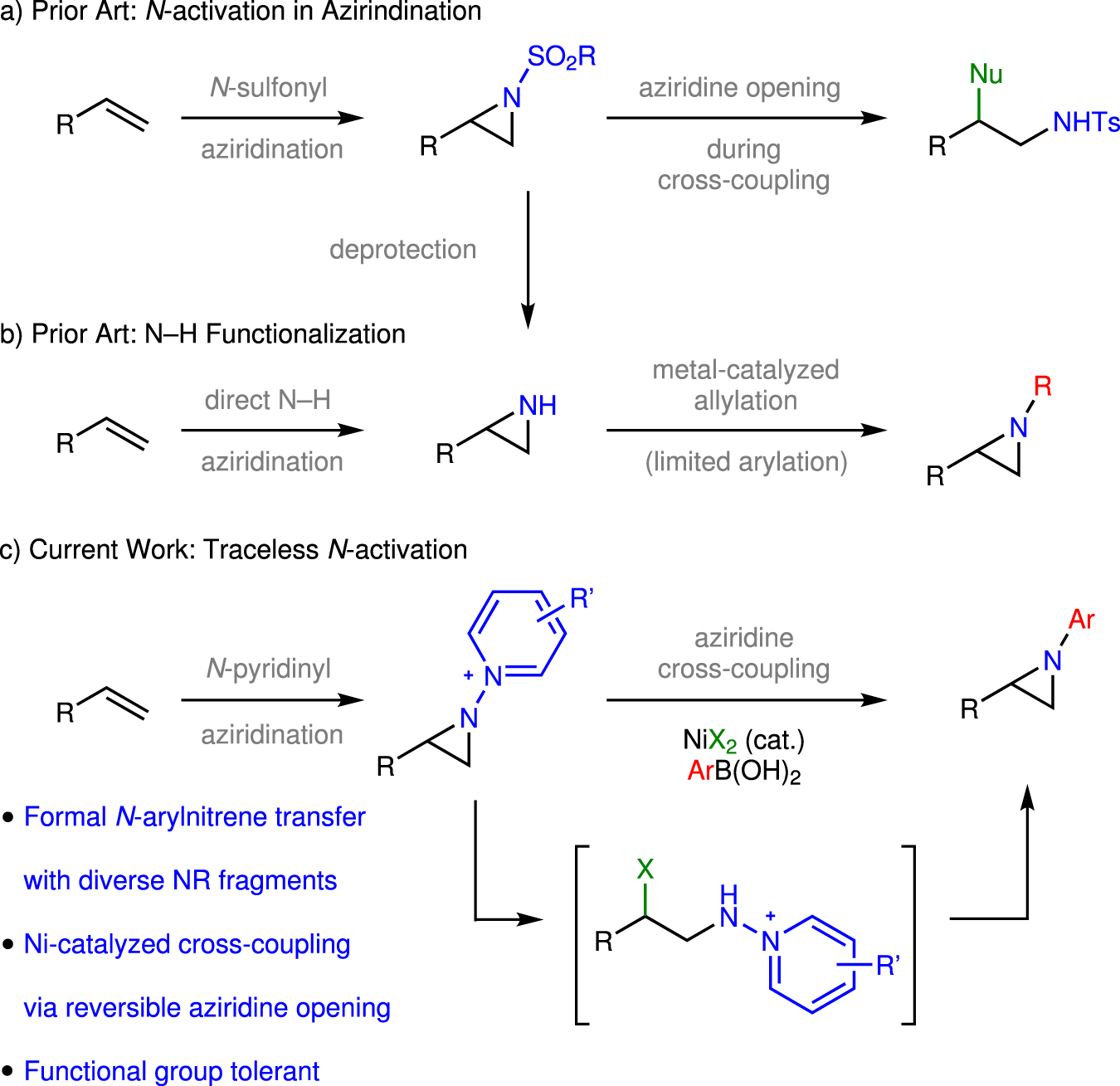
a Nitrene transfer to olefins provides access to aziridines but often requires the utilization of sulfonyl groups to activate the nitrogen. b N–H aziridines can be accessed directly from olefins and metal-catalyzed allylation methods enable functionalization of the N–H valence. c Here we advance N-pyridinium aziridines as platforms for C–N cross coupling to provide access to N-substituted aziridines.
