Fig. 1: Critical changes in the material composition of in situ forming implant formulations result in structural changes, increased payload, reduced erosion, and long-term effective drug delivery.
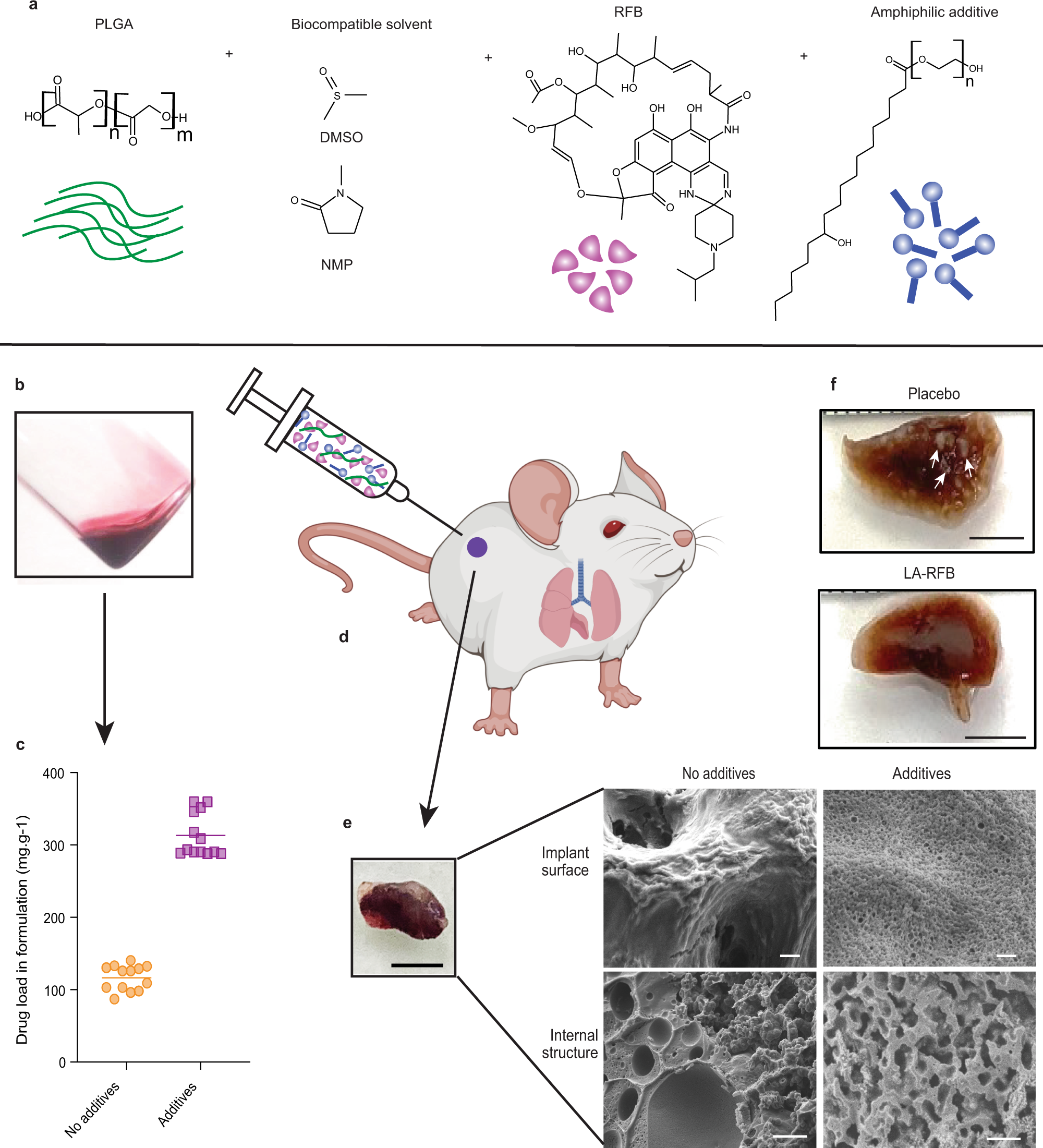
a Schematic showing LA-RFB composition consisting of PLGA as the biodegradable polymer, DMSO or NMP as a biocompatible solvent, RFB as an active pharmacological ingredient, and Kolliphor®HS 15 as an example of an additive. b The liquid injectable LA-RFB. c The drug load increases in LA-RFB formulations after addition of amphiphilic additives, n = 13 per group, mean value are shown, P = 0.0001, d The LA-RFB formulation can be administered subcutaneously and solidifies after injection. e A solidified implant of 50 μL LA-RFB, scale bar is 5 mm, and microphotographs of the implant surface and the internal structure without and without additives. Representative images are shown, three regions of each implant were scanned; three different implants were anlyzed for each formulation. Scale bar is 2 μm for the implant surface and 10 μm for the internal structure. f Formalin-fixed whole lung lobes of mice treated with placebo or LA-RFB prior to Mtb exposure. White lesions caused by Mtb are visible in lungs from mice which received placebo and are not present in mice which received LA-RFB (white arrows). Representative images of lung from six animals are shown. Parts of this figure were created using BioRender.com (2022). Source data for the panel c are provided as a Source data file.
