Table 1 Optimization of reaction conditionsa 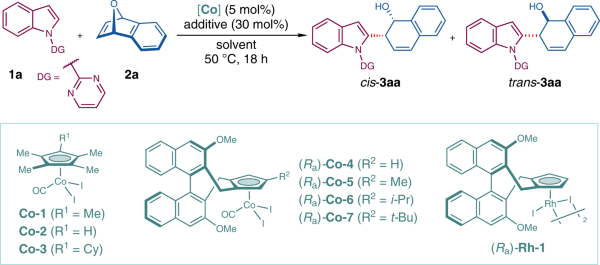
entry | [Co] | solvent | additive | yield (%)b | dr (cis-3aa/trans-3aa)c | ee of cis-3aa (%)c |
|---|---|---|---|---|---|---|
1 | Co-1 | TFE | CsOAc | 95 | 10:90 | – |
2 | Co-2 | TFE | CsOAc | 67 | 43:57 | – |
3 | Co-3 | TFE | CsOAc | 94 | 18:82 | – |
4d | (Ra)-Co-4 | TFE | CsOAc | 10 | 80:20 | 69 |
5 | (Ra)-Co-5 | TFE | CsOAc | 12 | 90:10 | >99 |
6 | (Ra)-Co-6 | TFE | CsOAc | 19 | 93:7 | >99 |
7 | (Ra)-Co-7 | TFE | CsOAc | 25 | 95:5 | >99 |
8 | (Ra)-Co-7 | TFE | CsOPiv | 39 | 96:4 | >99 |
9 | (Ra)-Co-7 | TFE | NaOPiv | 60 | 96:4 | >99 |
10 | (Ra)-Co-7 | TFE | HOPiv | 59 | 92:8 | >99 |
11 | (Ra)-Co-7 | HFIP | NaOPiv | 98 | 93:7 | 98 |
12 | (Ra)-Co-7 | TFE/HFIP (v/v = 1:1) | NaOPiv | 98 | 94:6 | 99 |
13 | (Ra)-Co-7 | TFE/HFIP (v/v = 3:1)e | NaOPiv | 98 | 95:5 | >99 |
14 | (Ra)-Rh-1 | TFE/HFIP (v/v = 3:1)e | NaOPiv | ND | – | – |

