Table 2 Condition optimization for enantiospecific reductive alkylative cyclization of 1,6-enynesa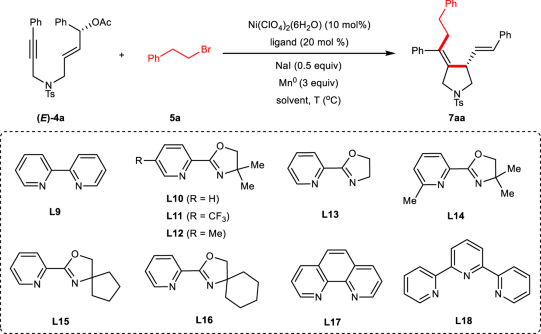
Entry | Ligand | Solvent | Yield (%) of 7aab | ee/es (%) of 7aac |
|---|---|---|---|---|
1 | L9 | DMA | 56 | 5/5 |
2 | L10 | DMA | 60 | 80/81 |
3d | L10 | DMA | 38 | 52/53 |
4e | L10 | DMA | 48 | 67/68 |
5 | L10 | THF | trace | – |
6 | L10 | DMF | 49 | 75/76 |
7 | L10 | DMSO | trace | – |
8 | L10 | DMA/THF(1/2) | 78 | 86/87 |
9 | L11 | DMA/THF(1/2) | 34 | 46/46 |
10 | L12 | DMA/THF(1/2) | 77 | 84/85 |
11 | L13 | DMA/THF(1/2) | 60 | 29/29 |
12 | L14 | DMA/THF(1/2) | trace | – |
13 | L15 | DMA/THF(1/2) | 78 | 91/92 |
14 | L16 | DMA/THF(1/2) | 63 | 84/85 |
15 | L17 | DMA/THF(1/2) | 43 | 8/8 |
16 | L18 | DMA/THF(1/2) | trace | – |
17f | L15 | DMA/THF(1/2) | 21 | 91/92 |
18g | L15 | DMA/THF(1/2) | trace | – |
19 | - | DMA/THF(1/2) | trace | – |

