Abstract
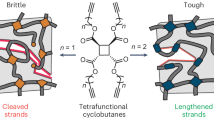
Enhancing the toughness while achieving triggerable degradation in single-network polymer systems without modifying their inherent chemical composition or network architecture remains a significant challenge. Here we demonstrate a smart end-linked polymer network that “self-strengthen” during use yet “self-destruct” upon certain stimuli. Embedding nonscissile cyclobutane-fused tetrahydrofuran mechanophores within the middle of end-linked polymer networks significantly enhances both toughness and degradability. Under mechanical stress, the force-coupled cycloreversion of these mechanophores releases concealed chain segments, enabling single-network materials to exhibit threefold toughness and tenfold tear energies compared to conventional counterparts. Additionally, ball-milling griding of the bulk material unveils acid-sensitive enol ether units, leading to a markedly improved degradation profile under acidic conditions. This dual effect—originating from the force-coupled cycloreversion of cyclobutane-fused tetrahydrofuran mechanophores—provides an ideal combination of superior mechanical performance and on-demand degradability.
Similar content being viewed by others
Data availability
All the other data supporting the findings of this study are available within the article and its Supplementary Information. All data are available from the corresponding author upon request.
References
Root, S. E., Savagatrup, S., Printz, A. D., Rodriquez, D. & Lipomi, D. J. Mechanical properties of organic semiconductors for stretchable, highly flexible, and mechanically robust electronics. Chem. Rev. 117, 6467–6499 (2017).
Zhao, X. et al. Soft materials by design: unconventional polymer networks give extreme properties. Chem. Rev. 121, 4309–4372 (2021).
Caruso, M. M. et al. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798 (2009).
Lloyd, E. M., Vakil, J. R., Yao, Y., Sottos, N. R. & Craig, S. L. Covalent mechanochemistry and contemporary polymer network chemistry: a marriage in the making. J. Am. Chem. Soc. 145, 751–768 (2023).
Wang, Z. J. & Gong, J. P. Mechanochemistry for on-demand polymer network materials. Macromolecules 58, 4–17 (2025).
Ramirez, A. L. B. et al. Mechanochemical strengthening of a synthetic polymer in response to typically destructive shear forces. Nat. Chem. 5, 757–761 (2013).
Zhang, H. et al. Mechanochromism and mechanical-force-triggered cross-linking from a single reactive moiety incorporated into polymer chains. Angew. Chem. Int. Ed. 55, 3040–3044 (2016).
Pan, Y. et al. A mechanochemical reaction cascade for controlling load-strengthening of a mechanochromic polymer. Angew. Chem. Int. Ed. 59, 21980–21985 (2020).
Seshimo, K. et al. Segmented polyurethane elastomers with mechanochromic and self-strengthening functions. Angew. Chem. Int. Ed. 60, 8406–8409 (2021).
Zheng, Y. et al. In situ and real-time visualization of mechanochemical damage in double-network hydrogels by prefluorescent probe via oxygen-relayed radical trapping. J. Am. Chem. Soc. 145, 7376–7389 (2023).
Wang, Z. J. et al. Rapid self-strengthening in double-network hydrogels triggered by bond scission. Nat. Mater. 24, 607–614 (2025).
Li, X. et al. Weak covalent bonds and mechanochemistry for synergistic self-strengthening of elastomers. J. Am. Chem. Soc. 147, 4357–4364 (2025).
Wang, S. et al. Facile mechanochemical cycloreversion of polymer cross-linkers enhances tear resistance. Science 380, 1248–1252 (2023).
Yokochi, H. et al. Sacrificial mechanical bond is as effective as a sacrificial covalent bond in increasing cross-linked polymer toughness. J. Am. Chem. Soc. 145, 23794–23801 (2023).
Wang, Z. et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 374, 193–196 (2021).
Wang, S. et al. Mechanism dictates mechanics: a molecular substituent effect in the macroscopic fracture of a covalent polymer network. J. Am. Chem. Soc. 143, 3714–3718 (2021).
Li, Y. et al. Azobenzene as a photoswitchable mechanophore. Nat. Chem. 16, 446–455 (2024).
Beech, H. K. et al. Reactivity-guided depercolation processes determine fracture behavior in end-linked polymer networks. ACS Macro Lett. 12, 1685–1691 (2023).
Herzog-Arbeitman, A. et al. Tetrafunctional cyclobutanes tune toughness via network strand continuity. Nat. Chem. https://doi.org/10.1038/s41557-025-01984-9 (2025).
Zheng, X. et al. Tuning the ultimate strain of single and double network gels through reactive strand extension. ACS Cent. Sci. 11, 1882–1891 (2025).
Yang, J. & Xia, Y. Mechanochemical generation of acid-degradable poly(enol ether)s. Chem. Sci. 12, 4389–4394 (2021).
Lin, Y., Kouznetsova, T. B. & Craig, S. L. Mechanically gated degradable polymers. J. Am. Chem. Soc. 142, 2105–2109 (2020).
Hsu, T.-G. et al. A polymer with “locked” degradability: superior backbone stability and accessible degradability enabled by mechanophore installation. J. Am. Chem. Soc. 142, 2100–2104 (2020).
Lin, Y., Kouznetsova, T. B., Chang, C.-C. & Craig, S. L. Enhanced polymer mechanical degradation through mechanochemically unveiled lactonization. Nat. Commun. 11, 4987 (2020).
Hsu, T.-G. et al. Mechanochemically accessing a challenging-to-synthesize depolymerizable polymer. Nat. Commun. 14, 225 (2023).
Liu, P. et al. Mechanically triggered on-demand degradation of polymers synthesized by radical polymerizations. Nat. Chem. 16, 1184–1192 (2024).
Li, Z., Zhang, X., Zhao, Y. & Tang, S. Mechanochemical backbone editing for controlled degradation of vinyl polymers. Angew. Chem. Int. Ed. 63, e202408225 (2024).
Zhang, X. et al. Polyethylene materials with tunable degradability by incorporating in-chain mechanophores. J. Am. Chem. Soc. 146, 24024–24032 (2024).
Bagheri, A. & Jin, J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 1, 593–611 (2019).
Hoyle, C. E. & Bowman, C. N. Thiol–Ene click chemistry. Angew. Chem. Int. Ed. 49, 1540–1573 (2010).
Rivlin, R. S. & Thomas, A. G. Rupture of rubber. I. Characteristic energy for tearing. J. Polym. Sci. 10, 291–318 (2003).
Fortman, D. J. et al. Approaches to sustainable and continually recyclable cross-linked polymers. ACS Sustainable Chem. Eng. 6, 11145–11159 (2018).
Rajasooriya, T., Ogasawara, H., Dong, Y., Mancuso, J. N. & Salaita, K. Force-triggered self-destructive hydrogels. Adv. Mater. 35, 2305544 (2023).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (52473097, 22401187) and the Fundamental Research Funds for the Central Universities (25X010202131). We thank Prof. G. Tong (SJTU) for the help with FTIR-ATR and rheological measurements, Prof. X. Yan (SJTU) for the technical help with tensile strength measurements.
Author information
Authors and Affiliations
Contributions
S.T. contributed to the conception and design of the experiments. Z.L. performed the experiments. S.T. and Z.L. cowrote the manuscript, and S.T. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Z., Tang, S. Cycloreversion-enhanced toughness and degradability in mechanophore-embedded end-linked polymer networks. Nat Commun (2026). https://doi.org/10.1038/s41467-025-68268-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-68268-1