Abstract
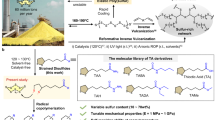
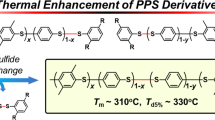
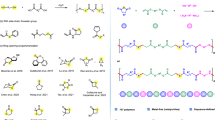
Elemental sulfur (S8), an abundant petroleum byproduct, is leveraged as a linchpin monomer in an organobase-catalyzed step-growth addition polymerization with dithiols and diacrylates at ambient temperature. This method enables the scalable synthesis of poly(ester disulfide)s—featuring alternating ester and disulfide linkages—with exceptional atom economy ( > 95% yield), Mn up to 42.0 kDa, and dual functionality: biodegradable ester units and stimuli-responsive disulfides. Mechanistic studies reveal a chemoselective three-component coupling involving S8 ring-opening, disulfide anion formation, and Michael addition, quantitatively generating symmetric and asymmetric disulfides in near-equimolar ratios. Thermal and mechanical characterizations of the poly(ester disulfide)s reveal programmable properties: High thermal stability (Td,5% = 248–281 °C), tunable phase behavior (amorphous Tg = −64 °C to semicrystalline Tm = 142 °C), and reductive degradation. By overcoming traditional limitations of harsh conditions and monomer scope, this strategy establishes S8 as a versatile feedstock for functional polymers, opening avenues for dynamic materials in biomedicine and environmental remediation.
Similar content being viewed by others
Data availability
Data supporting the findings of this study are available within the article (and its Supplementary information files). All data were available from the corresponding author upon request.
References
Zheng, N., Xu, Y., Zhao, Q. & Xie, T. Dynamic covalent polymer networks: a molecular platform for designing functions beyond chemical recycling and self-healing. Chem. Rev. 121, 1716–1745 (2021).
Lei, Z. et al. New advances in covalent network polymers via dynamic covalent chemistry. Chem. Rev. 124, 7829–7906 (2024).
Maes, S., Badi, N., Winne, J. M. & Du Prez, F. E. Taking dynamic covalent chemistry out of the lab and into reprocessable industrial thermosets. Nat. Rev. Chem. 9, 144–158 (2025).
Pal, S. et al. Recyclable surgical, consumer, and industrial adhesives of poly(α-lipoic acid). Science 385, 877–883 (2024).
Zhang, Q., Qu, D.-H., Feringa, B. L. & Tian, H. Disulfide-mediated reversible polymerization toward intrinsically dynamic smart materials. J. Am. Chem. Soc. 144, 2022–2033 (2022).
Deng, Y. et al. Acylhydrazine-based reticular hydrogen bonds enable robust, tough, and dynamic supramolecular materials. Sci. Adv. 8, eabk3286 (2022).
Bang, E.-K., Lista, M., Sforazzini, G., Sakai, N. & Matile, S. Poly(disulfide)s. Chem. Sci. 3, 1752–1763 (2012).
Mutlu, H. et al. Sulfur chemistry in polymer and materials science. Macromol. Rapid Commun. 40, 1800650 (2019).
Machado, T. O. et al. A renewably sourced, circular photopolymer resin for additive manufacturing. Nature 629, 1069–1074 (2024).
Zhang, X. & Waymouth, R. M. 1,2-Dithiolane-derived dynamic, covalent materials: cooperative self-assembly and reversible cross-linking. J. Am. Chem. Soc. 139, 3822–3833 (2017).
Lu, J. et al. Organ/cell-selective intracellular delivery of biologics via N-acetylated galactosamine-functionalized polydisulfide conjugates. J. Am. Chem. Soc. 146, 3974–3983 (2024).
Guo, J. et al. Synthesis of cationic cyclic oligo(disulfide)s via cyclo-depolymerization: a redox-responsive and potent antibacterial reagent. J. Am. Chem. Soc. 147, 6772–6785 (2025).
Yu, D. et al. Controllable star cationic poly(disulfide)s achieve genetically cascade catalytic therapy by delivering bifunctional fusion plasmids. Adv. Mater. 35, 2307190 (2023).
Huang, H. et al. Long-range electronic effect-promoted ring-opening polymerization of thioctic acid to produce biomimetic ionic elastomers for bioelectronics. CCS Chem. 6, 761–773 (2024).
Shi, C.-Y. et al. Robust and dynamic underwater adhesives enabled by catechol-functionalized poly(disulfides) network. Natl. Sci. Rev. 10, nwac139 (2023).
Du, T. et al. Controlled and regioselective ring-opening polymerization for poly(disulfide)s by anion-binding catalysis. J. Am. Chem. Soc. 145, 27788–27799 (2023).
Liu, Y., Jia, Y., Wu, Q. & Moore, J. S. Architecture-controlled ring-opening polymerization for dynamic covalent poly(disulfide)s. J. Am. Chem. Soc. 141, 17075–17080 (2019).
Gallizioli, C., Deglmann, P. & Plajer, A. J. Kinetically enhanced access to a dynamic polyester platform via sequence selective terpolymerisation of elemental sulphur. Angew. Chem. Int. Ed. 64, e202501337 (2025).
Zhang, Q. et al. Assembling a natural small molecule into a supramolecular network with high structural order and dynamic functions. J. Am. Chem. Soc. 141, 12804–12814 (2019).
Wang, B.-S. et al. Acid-catalyzed disulfide-mediated reversible polymerization for recyclable dynamic covalent materials. Angew. Chem. Int. Ed. 62, e202215329 (2023).
Albanese, K. R., Morris, P. T., Read de Alaniz, J., Bates, C. M. & Hawker, C. J. Controlled-radical polymerization of α-lipoic acid: a general route to degradable vinyl copolymers. J. Am. Chem. Soc. 145, 22728–22734 (2023).
Fang, J. et al. Electrochemical polymerization of 1,2-dithiolane derivatives at room temperature. Angew. Chem. Int. Ed. 64, e202506724 (2025).
Kandemir, D., Luleburgaz, S., Gunay, U. S., Durmaz, H. & Kumbaraci, V. Ultrafast poly(disulfide) synthesis in the presence of organocatalysts. Macromolecules 55, 7806–7816 (2022).
Mu, Y. et al. Polymerization-induced self-coacervation of alternating poly(disulfide)s via ring-opening reaction-mediated polycondensation of cyclic thiosulfinate and dithiol. Macromolecules 58, 74–86 (2025).
Kim, S., Wittek, K. I. & Lee, Y. Synthesis of poly(disulfide)s with narrow molecular weight distributions via lactone ring-opening polymerization. Chem. Sci. 11, 4882–4886 (2020).
Frandsen, M. et al. Automated solid-phase oligo(disulfide) synthesis. Angew. Chem. Int. Ed. 62, e202303170 (2023).
Mondal, A. et al. Cascade initiation of ring opening polymerization for dynamic covalent poly(disulfide)s: one-step double modification and copoly(disulfide) synthesis by living polymerization. Macromolecules 57, 11350–11360 (2024).
Penczek, S., Cypryk, M., Pretula, J., Kaluzynski, K. & Lewinski, P. Elemental sulfur and cyclic sulfides. Homo- and copolymerizations. Kinetics, thermodynamics and DFT analysis. Prog. Polym. Sci. 152, 101818 (2024).
Zhang, R., Nie, T., Fang, Y., Huang, H. & Wu, J. Poly(disulfide)s: from synthesis to drug delivery. Biomacromolecules 23, 1–19 (2022).
Kang, K.-S. et al. Sulfenyl Chlorides: An alternative monomer feedstock from elemental sulfur for polymer synthesis. J. Am. Chem. Soc. 144, 23044–23052 (2022).
Lee, T., Dirlam, P. T., Njardarson, J. T., Glass, R. S. & Pyun, J. Polymerizations with elemental sulfur: from petroleum refining to polymeric materials. J. Am. Chem. Soc. 144, 5–22 (2022).
Manjunatha, B. R. et al. Sequence control in sulphur-containing ring-opening co- and terpolymerisations. Angew. Chem. Int. Ed. 64, e202507243 (2025).
Lim, J., Pyun, J. & Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 54, 3249–3258 (2015).
Yang, H. et al. Anionic hybrid copolymerization of sulfur with acrylate: strategy for synthesis of high-performance sulfur-based polymers. J. Am. Chem. Soc. 145, 14539–14547 (2023).
Chung, W. J. et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 5, 518 (2013).
Gallizioli, C., Battke, D., Schlaad, H., Deglmann, P. & Plajer, A. J. Ring-opening terpolymerisation of elemental sulfur waste with propylene oxide and carbon disulfide via lithium catalysis. Angew. Chem. Int. Ed. 63, e202319810 (2024).
Deng, Y. et al. Converting inorganic sulfur into degradable thermoplastics and adhesives by copolymerization with cyclic disulfides. Nat. Commun. 15, 3855 (2024).
Jia, J. et al. Photoinduced inverse vulcanization. Nat. Chem. 14, 1249–1257 (2022).
Zhang, J. et al. Cyclization polymerization of elemental sulfur and diisocyanate: new polymerization toward high-performance polymer. Angew. Chem. Int. Ed. 64, e202502207(2025).
Zhang, J. et al. Sulfur conversion to multifunctional poly(O-thiocarbamate)s through multicomponent polymerizations of sulfur, diols, and diisocyanides. J. Am. Chem. Soc. 143, 3944–3950 (2021).
Marshall, C. M. et al. Synthesis of polycyclic olefinic monomers from norbornadiene for inverse vulcanization: structural and mechanistic consequences. J. Am. Chem. Soc. 146, 24061–24074 (2024).
He, L., Zhao, H. & Theato, P. No heat, no light—the future of sulfur polymers prepared at room temperature is bright. Angew. Chem. Int. Ed. 57, 13012–13014 (2018).
Fan, J. et al. Inverse vulcanization of aziridines: enhancing polysulfides for superior mechanical strength and adhesive performance. Angew. Chem. Int. Ed. 64, e202418764 (2025).
Huang, H. et al. Step-growth polymerization of aziridines with elemental sulfur: easy access to linear polysulfides and their use as recyclable adhesives. Angew. Chem. Int. Ed. 63, e202318919 (2024).
Tang, H. et al. Direct synthesis of thioesters from feedstock chemicals and elemental sulfur. J. Am. Chem. Soc. 145, 5846–5854 (2023).
Zheng, B. et al. Structural evolution during inverse vulcanization. Nat. Commun. 15, 5507 (2024).
Tian, T., Hu, R. & Tang, B. Z. Room temperature one-step conversion from elemental sulfur to functional polythioureas through catalyst-free multicomponent polymerizations. J. Am. Chem. Soc. 140, 6156–6163 (2018).
Cao, W., Dai, F., Hu, R. & Tang, B. Z. Economic sulfur conversion to functional polythioamides through catalyst-free multicomponent polymerizations of sulfur, acids, and amines. J. Am. Chem. Soc. 142, 978–986 (2020).
Huang, Y., Yu, Y., Hu, R. & Tang, B. Z. Multicomponent polymerizations of elemental sulfur, CH2Cl2, and aromatic amines toward chemically recyclable functional aromatic polythioureas. J. Am. Chem. Soc. 146, 14685–14696 (2024).
Chao, J.-Y. et al. Controlled disassembly of elemental sulfur: an approach to the precise synthesis of polydisulfides. Angew. Chem. Int. Ed. 61, e202115950 (2022).
Ottou, W. N., Sardon, H., Mecerreyes, D., Vignolle, J. & Taton, D. Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 56, 64–115 (2016).
Nair, D. P. et al. The thiol-Michael addition click reaction: a powerful and widely used tool in materials chemistry. Chem. Mater. 26, 724–744 (2014).
Yang, H., Zhang, J., Huang, W. & Zhang, G. Transforming element sulfur to high performance closed-loop recyclable polymer via proton transfer enabled anionic hybrid copolymerization. Angew. Chem. Int. Ed. 64, e202414244 (2025).
Bao, J. et al. On the mechanism of the inverse vulcanization of elemental sulfur: structural characterization of poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 145, 12386–12397 (2023).
Wu, Z. & Pratt, D. A. Radical substitution provides a unique route to disulfides. J. Am. Chem. Soc. 142, 10284–10290 (2020).
Zhang, Q., Li, Y., Zhang, L. & Luo, S. Catalytic asymmetric disulfuration by a chiral bulky three-component Lewis acid-base. Angew. Chem. Int. Ed. 60, 10971–10976 (2021).
Zhou, F. et al. Generation of perthiyl radicals for the synthesis of unsymmetric disulfides. Nat. Commun. 16, 23 (2025).
Huang, P., Wang, P., Tang, S., Fu, Z. & Lei, A. Electro-oxidative S−H/S−H cross-coupling with hydrogen evolution: facile access to unsymmetrical disulfides. Angew. Chem. Int. Ed. 57, 8115–8119 (2018).
Jin, S. et al. Elemental-sulfur-enabled divergent synthesis of disulfides, diselenides, and polythiophenes from β-CF3−1,3-enynes. Angew. Chem. Int. Ed. 60, 881–888 (2021).
Ong, C. L., Titinchi, S., Juan, J. C. & Khaligh, N. G. An overview of recent advances in the synthesis of organic unsymmetrical disulfides. Helv. Chim. Acta. 104, e2100053 (2021).
Schmitt, C. W. et al. A critical review on the sustainability of inverse vulcanised polymers. RSC Sustain. 3, 4190–4227 (2025).
Tveryanovich, Y. S., Pankin, D. V., Sukhanov, M. V. & Churbanov, M. F. Isotope effect in Raman scattering spectra of 32S8–34S8 solid mixtures. Optik 240, 166861 (2021).
Duarte, M. E., Huber, B., Theato, P. & Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 11, 241–248 (2020).
Acknowledgements
We gratefully acknowledge the financial support of the Natural Science Foundation of Zhejiang Province (LR26E030001, received by Chengjian Zhang) and the National Natural Science Foundation of China (52373014, received by Chengjian Zhang).
Author information
Authors and Affiliations
Contributions
Y.S. carried out most of the experiments and wrote the draft. Y.C. carried out the analysis of polymer structure. X.L. carried out the analysis of the polymerization mechanism. C.Z. conceived, designed, and directed the investigation and revised the manuscript. X.Z. directed the investigation and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hatice Mutlu, Alex Plajer, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Y., Cao, Y., Liu, X. et al. Synthesis of poly(ester disulfide)s from S8-involved step-growth addition polymerization at ambient temperature. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68963-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68963-7