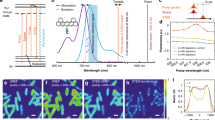
Abstract
Vibrational spectroscopy is a powerful tool for spectral imaging of biological samples, thanks to its narrow bandwidth (10 cm⁻¹) compared to fluorescence. Single-molecule vibrational spectroscopy has been demonstrated with near-field amplification as in surface-enhanced Raman spectroscopy or fluorescence detection as in stimulated Raman excited fluorescence and bond-selective fluorescence-detected infrared-excited spectro-microscopy. However, these methods often require elaborate sample preparation or sometimes generate background signals when unintended processes lead to fluorescence emission. In response to these issues, we developed electronic resonance stimulated Raman scattering (ER-SRS) to achieve single-molecule sensitivity in far-field vibrational microscopy without relying on fluorescence detection. ER-SRS has encountered difficulties due to large electronic backgrounds. To overcome this, we employed Raman-amplified nonfluorescent molecular probe (RANMP) alongside our synchronously pumped, independently tunable double optical parametric oscillators for effective optimization of the signal-to-background ratio. The optimization of probe and light source allowed us to successfully detect ER-SRS signal from single particles in solution and from single molecules embedded in polymer matrix. ER-SRS combined with RANMP provides single-molecule sensitivity without fluorescence detection, enabling applications in biological and chemical imaging, particularly in multiplexed imaging.
Similar content being viewed by others
Data availability
The data supporting the findings of this study consist primarily of spectroscopy datasets. Due to the absence of a standardized public repository that supports these data formats in a reusable manner, the data are not deposited in a public database. The data that support the findings of this study are available from the corresponding author upon request.
Code availability
The codes that support the plots and data analysis within this paper are available from the corresponding author upon request.
References
Ha, T. et al. Probing the interaction between two single molecules: fluorescence resonance energy transfer between a single donor and a single acceptor. Proc. Natl. Acad. Sci. USA 93, 6264–6268 (1996).
Yildiz, A. et al. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 300, 2061–2065 (2003).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 (2006).
Manley, S. et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 (2008).
Bintu, B. et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 362, eaau1783 (2018).
Metzker, M. L. Sequencing technologies—the next generation. Nat. Rev. Genet. 11, 31–46 (2010).
Sigal, Y. M. et al. Visualizing and discovering cellular structures with super-resolution microscopy. Science 361, 880–887 (2018).
Balzarotti, F. et al. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 355, 606–612 (2017).
Weisenburger, S. et al. Cryogenic optical localization provides 3D protein structure data with Angstrom resolution. Nat. Methods 14, 141–144 (2017).
Reinhardt, S. C. M. et al. Ångström-resolution fluorescence microscopy. Nature 617, 711–716 (2023).
Chen, K. H. et al. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Moffitt, J. R., Lundberg, E. & Heyn, H. The emerging landscape of spatial profiling technologies. Nat. Rev. Genet. 23, 741–759 (2022).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 3rd edn (Springer, 2011).
Nie, S. & Emory, S. R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275, 1102–1106 (1997).
Langer, J. et al. Present and future of surface-enhanced Raman scattering. ACS Nano 14, 28–117 (2020).
Laing, S., Gracie, K. & Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 45, 1901–1918 (2016).
Jimenez de Aberasturi, D. et al. Surface enhanced Raman scattering encoded gold nanostars for multiplexed cell discrimination. Chem. Mater. 28, 6779–6790 (2016).
Kang, H. et al. Near-Infrared SERS nanoprobes with plasmonic Au/Ag hollow-shell assemblies for in vivo multiplex detection. Adv. Funct. Mater. 23, 3719–3727 (2013).
Wei, L. et al. Super-multiplex vibrational imaging. Nature 544, 465–470 (2017).
Xiong, H. et al. Stimulated Raman excited fluorescence spectroscopy and imaging. Nat. Photonics 13, 412–417 (2019).
Xiong, H. et al. Stimulated Raman excited fluorescence spectroscopy of visible dyes. J. Phys. Chem. Lett. 10, 3563–3570 (2019).
Xiong, H. & Min, W. Combining the best of two worlds: stimulated Raman excited fluorescence. J. Chem. Phys. 153, 210901 (2020).
Wang, H. et al. Bond-selective fluorescence imaging with single-molecule sensitivity. Nat. Photonics 17, 846–855 (2023).
Shi, L. et al. Electronic resonant stimulated Raman scattering micro-spectroscopy. J. Phys. Chem. B 122, 9218–9224 (2018).
Cho, S. J. et al. A-D-A type semiconducting small molecules with bis(alkylsulfanyl)methylene substituents and control of charge polarity for organic field-effect transistors. ACS Appl. Mater. Interfaces 12, 41842–41851 (2020).
Cho, E. et al. Impact of secondary donor units on the excited-state properties and thermally activated delayed fluorescence (TADF) efficiency of pentacarbazole-benzonitrile emitters. J. Chem. Phys. 153, 144708 (2020).
Nguyen, V.-N. et al. An emerging molecular design approach to heavy-atom-free photosensitizers for enhanced photodynamic therapy under hypoxia. J. Am. Chem. Soc. 141, 16243–16248 (2019).
Nguyen, V.-N. et al. Design and synthesis of efficient heavy-atom-free photosensitizers for photodynamic therapy of cancer. Chem. Commun. 56, 11489–11492 (2020).
Jeong, J.-E. et al. Resonant Raman-active polymer dot barcodes for multiplex cell mapping. ACS Nano 17, 4800–4812 (2023).
Kwon, J., Elgawish, M. S. & Shim, S.-H. Bleaching-resistant super-resolution fluorescence microscopy. Adv. Sci. 9, 2101817 (2022).
Wennmalm, S. & Rigler, R. On death numbers and survival times of single dye molecules. J. Phys. Chem. B 103, 2516–2519 (1999).
Mutch, S. A. et al. Deconvolving single-molecule intensity distributions for quantitative microscopy measurements. Biophys. J. 92, 2926–2943 (2007).
Gensch, T. et al. Single molecule blinking and photobleaching separated by wide-field fluorescence microscopy. J. Phys. Chem. A 109, 6652–6658 (2005).
Kocheril, P. A. et al. Nitrile vibrational lifetimes as probes of local electric fields. J. Phys. Chem. Lett. 15, 5306–5314 (2024).
Lu, F.-K. et al. Label-free DNA imaging in vivo with stimulated Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 112, 11624–11629 (2015).
Fu, D. et al. In vivo metabolic fingerprinting of neutral lipids with hyperspectral stimulated Raman scattering microscopy. J. Am. Chem. Soc. 136, 8820–8828 (2014).
Santra, D. C. et al. Charge-transfer-induced fluorescence quenching of anthracene derivatives and selective detection of picric acid. Chem. Eur. J. 22, 2012–2019 (2016).
Bu, L. et al. An AIE and ICT based NIR florescent probe for cysteine and homocysteine. Dyes Pigm 136, 724–731 (2017).
Bednarik, S. et al. Tuning electron-accepting properties of phthalocyanines for charge transfer processes. Inorg. Chem. 63, 8799–8806 (2024).
Cordes, T. et al. Mechanisms and advancement of antifading agents for fluorescence microscopy and single-molecule spectroscopy. Phys. Chem. Chem. Phys. 13, 6699–6709 (2011).
Yang, Y., Bai, X. & Hu, F. Photoswitchable polyynes for multiplexed stimulated Raman scattering microscopy with reversible light control. Nat. Commun. 15, 2578 (2024).
Chen, Z. et al. Multicolor live-cell chemical imaging by isotopically edited alkyne vibrational palette. J. Am. Chem. Soc. 136, 8027–8033 (2014).
Hu, F. et al. Supermultiplexed optical imaging and barcoding with engineered polyynes. Nat. Methods 15, 194–200 (2018).
Miao, Y. et al. 9-Cyanopyronin probe palette for super-multiplexed vibrational imaging. Nat. Commun. 12, 4518 (2021).
Marx, V. Method of the Year: spatially resolved transcriptomics. Nat. Methods 18, 9–14 (2021).
Method of the Year 2024: spatial proteomics. Nat. Methods 21, 2195–2196 (2024).
Schueder, F. et al. Unraveling cellular complexity with transient adapters in highly multiplexed super-resolution imaging. Cell 187, 1769–1784 (2024).
Unterauer, E. M. et al. Spatial proteomics in neurons at single-protein resolution. Cell 187, 1785–1800 (2024).
Shi, L., Hu, F. & Min, W. Optical mapping of biological water in single live cells by stimulated Raman excited fluorescence microscopy. Nat. Commun. 10, 4764 (2019).
Acknowledgements
This work was supported by Samsung Science and Technology Foundation SSTF-BA2201-07 and a Korea University Grant for S.-H.S. This work was also supported by grants from the National Research Foundation of Korea (RS-2022-NR069080 for S.P., RS-2024-00334832 for H.Y.W. and RS-2024-00357947 for S.-H.S.)
Author information
Authors and Affiliations
Contributions
H.Y.W. and S.-H.S. conceived and designed the research. S.O. built the ER-SRS set-up and performed all the SRS spectroscopy and microscopy experiments. Y.E. and A.T. prepared polymer dots and film samples. S.O. and Y.E. collected and analyzed the data. H.Y.W. and S.-H.S. supervised the experiments. S.P. supervised theoretical and quantum chemical calculations and analyzed the results. H.Y.K. performed theoretical and quantum chemical calculations. The manuscript was written by S.O. and S.-H.S. with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Lu Wei and the other anonymous reviewers for their contribution to the peer review of this work. [A peer review file is available.]
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oh, S., Eom, Y., Kim, H.Y. et al. Fluorescence-free single-molecule microscopy via electronic resonance stimulated Raman scattering. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69348-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69348-6