Abstract
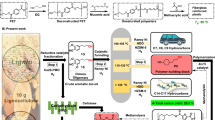
Transitioning to a circular plastics economy will require use of renewable feedstocks, energy-efficient processes, and closed-loop recyclable polymers, such as polyesters. A key challenge lies in sustainably sourcing monomers used to make recyclable polyesters. This work presents a catalytic platform utilizing earth-abundant Cu(x)Ca(1-x)O mixed metal oxides for the oxidative dehydrocyclization of bio-based diols to lactones, which are advantaged for energy-efficient ring-opening polymerization. Operating below 200 °C, at ambient pressure, and without solvent, the process uses air as the sole oxidant, achieving high yields of lactones across a broad substrate scope of C4-8 diols in the liquid phase. The oxidative dehydrocyclization reaction is thermodynamically downhill due to water formation and energy-efficient compared to incumbent, non-redox pathways utilized in fossil carbon-based industrial processes for lactone production. Mechanistic studies reveal facile redox cycling of Cu2+-O(Ca2+)-Cu2+ interfacial sites unique to the developed catalyst. Techno-economic analysis and life cycle assessment estimate 40% lower energy demand and 15% lower GHG intensity per mass of butyrolactone produced compared to the fossil carbon-based route. Liquid-phase oxidative dehydrocyclization offers a promising approach for scalable lactone production from renewable, bio-based diols to enable circular polyesters.
Similar content being viewed by others
Data availability
All data are available from the corresponding author, Gregg T. Beckham (gregg.beckham@nlr.gov), upon request. Additionally, supplementary experimental details regarding the reaction setup, additional experimental results including NMR spectra, product inhibition studies, IR spectra, ICP-OES results, process-flow diagram and TEA/LCA details. Supplementary data tables also contain numerical data from Fig. 3 and Fig. 6 of main text.
References
Karali, N., Khanna, N. & Shah, N. Climate Impact of Primary Plastic Production (Lawrence Berkeley National Laboratory, 2024).
Aarsen, C. V., Liguori, A., Mattsson, R., Sipponen, M. H. & Hakkarainen, M. Designed to degrade: tailoring polyesters for circularity. Chem. Rev. 124, 8473–8515 (2024).
Hong, M. & Chen, E. Y. X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 8, 42–49 (2016).
Tang, X. & Chen, E. Y. X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 9, 2345 (2018).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Shi, C. et al. Design principles for intrinsically circular polymers with tunable properties. Chem 7, 2896–2912 (2021).
Li, X.-L. et al. Dual recycling of depolymerization catalyst and biodegradable polyester that markedly outperforms polyolefins. Angew. Chem. Int. Ed. 62, e202303791 (2023).
Zhang, Z., Gowda, R. R. & Chen, E. Y. X. Chemosynthetic P4HB: a ten-year journey from a “non-polymerizable” monomer to a high-performance biomaterial. Acc. Mater. Res. 5, 1340–1352 (2024).
Zhou, L. et al. Chain-end controlled depolymerization selectivity in α,α-disubstituted propionate PHAs with dual closed-loop recycling and record-high melting temperature. J. Am. Chem. Soc. 146, 29895–29904 (2024).
Kim, M. S. et al. A review of biodegradable plastics: chemistry, applications, properties, and future research needs. Chem. Rev. 123, 9915–9939 (2023).
Lowe, J. R., Martello, M. T., Tolman, W. B. & Hillmyer, M. A. Functional biorenewable polyesters from carvone-derived lactones. Polym. Chem. 2, 702–708 (2011).
Cortright, R. D., Davda, R. R. & Dumesic, J. A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 418, 964–967 (2002).
He, J. et al. New catalytic strategies for α,ω-diols production from lignocellulosic biomass. Faraday Discuss. 202, 247–267 (2017).
Burt, S. P. et al. Production of 1,6-hexanediol from tetrahydropyran-2-methanol by dehydration–hydration and hydrogenation. Green. Chem. 19, 1390–1398 (2017).
Huang, K. et al. Conversion of furfural to 1,5-pentanediol: process synthesis and analysis. ACS Sustain. Chem. Eng. 5, 4699–4706 (2017).
Eagan, N. M., Kumbhalkar, M. D., Buchanan, J. S., Dumesic, J. A. & Huber, G. W. Chemistries and processes for the conversion of ethanol into middle-distillate fuels. Nat. Rev. Chem. 3, 223–249 (2019).
Stadler, B. M., Wulf, C., Werner, T., Tin, S. & de Vries, J. G. Catalytic approaches to monomers for polymers based on renewables. ACS Catal. 9, 8012–8067 (2019).
Kiani, D. et al. Production of bio-based lactones as monomers for circular polymers. Nat. Rev. Chem. 9, 749–765 (2025).
Krishna, S. H. et al. Oxygenated commodity chemicals from chemo-catalytic conversion of biomass derived heterocycles. AIChE J. 64, 1910–1922 (2018).
Cargill. Bio-based coatings, https://www.cargill.com/bioindustrial/coatings (2025).
ACS. Cargill to build biobased 1,4-butanediol plant, https://pubs.acs.org/doi/10.1021/cen-09922-buscon2 (2021).
Gantrade. Bio-Based 1,4-Butanediol (BDO), https://www.gantrade.com/products/bio-based-14-butanediol-bdo (2025).
Geno. Bio-based BDO, https://www.genomatica.com/products/ (2025).
Tan, X. et al. Lactonization of diols over highly efficient metal-based catalysts. ChemSusChem n/a, e202400909 (2024).
Ichikawa, N., Sato, S., Takahashi, R., Sodesawa, T. & Inui, K. Dehydrogenative cyclization of 1,4-butanediol over copper-based catalyst. J. Mol. Catal. A Chem. 212, 197–203 (2004).
Huang, J., Dai, W.-L., Li, H. & Fan, K. Au/TiO2 as high efficient catalyst for the selective oxidative cyclization of 1,4-butanediol to γ-butyrolactone. J. Catal. 252, 69–76 (2007).
Huang, J., Wang, Y., Zheng, J., Dai, W.-L. & Fan, K. Influence of support surface basicity and gold particle size on catalytic activity of Au/γ-AlOOH and Au/γ-Al2O3 catalyst in aerobic oxidation of α,ω-diols to lactones. Appl. Catal. B Environ. 103, 343–350 (2011).
Huang, Z. et al. Hydrogenation of γ-butyrolactone to 1,4-butanediol over CuCo/TiO2 bimetallic catalysts. ACS Catal. 7, 8429–8440 (2017).
Schwarz, W., Schossig, J., Rossbacher, R., Pinkos, R. & Höke, H. In Ullmann’s Encyclopedia of Industrial Chemistry 1–7 (Wiley, 2019).
Jochen, H. et al. Dehydrogenation of 1,4-butanediol to γ-butyrolactone. USA patent (1999).
Touchy, A. S. & Shimizu, K. -i Acceptorless dehydrogenative lactonization of diols by Pt-loaded SnO2 catalysts. RSC Adv. 5, 29072–29075 (2015).
Tana, T. et al. Non-plasmonic metal nanoparticles as visible light photocatalysts for the selective oxidation of aliphatic alcohols with molecular oxygen at near ambient conditions. Chem. Commun. 52, 11567–11570 (2016).
Torrubia, J., Valero, A. & Valero, A. Energy and carbon footprint of metals through physical allocation. Implications for energy transition. Resour. Conserv. Recycling 199, 107281 (2023).
Han, L. et al. Multifunctional high-entropy materials. Nat. Rev. Mater. 9, 846–865 (2024).
Zhong, W., Liu, H., Bai, C., Liao, S. & Li, Y. Base-free oxidation of alcohols to esters at room temperature and atmospheric conditions using nanoscale Co-based catalysts. ACS Catal. 5, 1850–1856 (2015).
Tang, D. et al. Aerobic oxidative lactonization of diols at room temperature over defective titanium-based oxides in water. J. Catal. 418, 237–246 (2023).
Bagley, M. C., Lin, Z., Phillips, D. J. & Graham, A. E. Barium manganate in microwave-assisted oxidation reactions: synthesis of lactones by oxidative cyclization of diols. Tetrahedron Lett. 50, 6823–6825 (2009).
Bhanushali, J. T. et al. Tailoring the catalytic activity of basic mesoporous Cu/CeO2 catalyst by Al2O3 for selective lactonization and dehydrogenation of 1,4-butanediol to γ-butyrolactone. Catal. Commun. 143, 106049 (2020).
Mitran, G., Nguyen, T. L. P. & Seo, D.-K. Effect of solvent, in the sol–gel synthesis of CoAl2O4, on the structure and catalytic properties in 1,4-butanediol dehydrocyclization. React. Chem. Eng. 8, 1901–1913 (2023).
Bhatia, A., Kannan, M. & Muthaiah, S. Ruthenium-promoted acceptorless and oxidant-free lactone synthesis in aqueous medium. Synlett 30, 721–725 (2019).
Liu, H., Jiang, Y., Zhao, H. & Hou, Z. Preparation of highly dispersed Cu catalysts from hydrotalcite precursor for the dehydrogenation of 1,4-butanediol. J. Ind. Eng. Chem. 102, 251–259 (2021).
Schweitzer, N. M., Gounder, R. & Rioux, R. M. Addressing Rigor and Reproducibility in Thermal, Heterogeneous Catalysis (1.00). Zenodo https://doi.org/10.5281/zenodo.8029159 (2023).
Kiani, D. & Wachs, I. E. Practical considerations for understanding surface reaction mechanisms involved in heterogeneous catalysis. ACS Catal. 14, 16770–16784 (2024).
Danks, A. E., Hall, S. R. & Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 3, 91–112 (2016).
Kiani, D. & Wachs, I. E. The conundrum of “Pair Sites” in Langmuir–Hinshelwood reaction kinetics in heterogeneous catalysis. ACS Catal. 14, 10260–10270 (2024).
Kiani, D., Ibrahim, F., Hayden, S., Hermans, I. & Beckham, G. T. Understanding the origin of negative temperature dependence and activity of N-coordinated cobalt sites during ethylene dimerization. Appl. Catal. B Environ. Energy 365, 124952 (2025).
Madon, R. J. & Boudart, M. Experimental criterion for the absence of artifacts in the measurement of rates of heterogeneous catalytic reactions. Ind. Eng. Chem. Fundam. 21, 438–447 (1982).
Li, K. & Xue, D. Estimation of electronegativity values of elements in different valence states. J. Phys. Chem. A 110, 11332–11337 (2006).
Di Castro, D. et al. Raman spectroscopy study of the interface structure in (CaCuO2)n/(SrTiO3)m superlattices. Appl. Phys. Lett. 103, 191903 (2013).
Kan, D., Yamanaka, A., Terashima, T. & Takano, M. Preparation and optical properties of single-crystalline CaCuO2 thin films with infinite layer structure. Phys. C Superconductivity 412-414, 298–302 (2004).
Fouad, O. A. et al. Synthesis, characterization and application of some nanosized mixed metal oxides as high heat resistant pigments: Ca2CuO3, Ca3Co2O6, and NiSb2O6. J. Alloy. Compd. 537, 165–170 (2012).
Chakrabarti, A. & Wachs, I. E. Molecular structure–reactivity relationships for olefin metathesis by Al2O3-supported surface MoOx sites. ACS Catal. 8, 949–959 (2018).
Kiani, D. & Baltrusaitis, J. A spectroscopic study of supported-phosphate-catalysts (SPCs): evidence of surface-mediated hydrogen-transfer. ChemCatChem 13, 2064–2073 (2021).
Bravo-Suárez, J. J., Subramaniam, B. & Chaudhari, R. V. Ultraviolet–visible spectroscopy and temperature-programmed techniques as tools for structural characterization of Cu in CuMgAlOx mixed metal oxides. J. Phys. Chem. C. 116, 18207–18221 (2012).
Angelici, C., Velthoen, M. E. Z., Weckhuysen, B. M. & Bruijnincx, P. C. A. Effect of preparation method and CuO promotion in the conversion of ethanol into 1,3-butadiene over SiO2–MgO catalysts. ChemSusChem 7, 2505–2515 (2014).
Smeets, P. J., Groothaert, M. H. & Schoonheydt, R. A. Cu based zeolites: a UV–vis study of the active site in the selective methane oxidation at low temperatures. Catal. Today 110, 303–309 (2005).
Giordanino, F. et al. Characterization of Cu-exchanged SSZ-13: a comparative FTIR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-β with similar Si/Al and Cu/Al ratios. Dalton Trans. 42, 12741–12761 (2013).
Lezcano-Gonzalez, I. et al. Determining the storage, availability and reactivity of NH3 within Cu-Chabazite-based Ammonia Selective Catalytic Reduction systems. Phys. Chem. Chem. Phys. 16, 1639–1650 (2014).
Gu, M. et al. Structure–activity relationships of copper- and potassium-modified iron oxide catalysts during reverse water–gas shift reaction. ACS Catal. 11, 12609–12619 (2021).
Wang, B., Jin, M., An, H., Guo, Z. & Lv, Z. Hydrogenation performance of acetophenone to 1-phenylethanol on highly active nano Cu/SiO2 catalyst. Catal. Lett. 150, 56–64 (2020).
Fierro, G. et al. A study of anomalous temperature-programmed reduction profiles of Cu2O, CuO, and CuO-ZnO catalysts. J. Catal. 148, 709–721 (1994).
Kim, J. Y., Rodriguez, J. A., Hanson, J. C., Frenkel, A. I. & Lee, P. L. Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides. J. Am. Chem. Soc. 125, 10684–10692 (2003).
Villarroel-Rocha, J. & Gil, A. Modeling the temperature-programmed reduction of metal oxide catalysts by considering the particle-size distribution effect. Chem. Eng. J. 487, 150722 (2024).
Klissurski, D. & Rives, V. High-temperature superconductors in catalysis. Appl. Catal. A Gen. 109, 1–44 (1994).
Satam, C. C., Daub, M. & Realff, M. J. Techno-economic analysis of 1,4-butanediol production by a single-step bioconversion process. Biofuels Bioprod. Bioref. 13, 1261–1273 (2019).
United States Census Bureau USA Trade Import and Export Data, https://usatrade.census.gov/ (2025).
van Bree, R. A. B. & Kroes, G. J. O2 dissociation on Cu(111) dynamics on a novel screened hybrid van der Waals DFT potential energy surface. J. Phys. Chem. C. 128, 19182–19196 (2024).
Junell, P., Ahonen, M., HirsimÄKi, M. & Valden, M. Influence of surface modification on the adsorption dynamics of O2 on Cu{100}. Surf. Rev. Lett. 11, 457–461 (2004).
Hodgson, A., Lewin, A. K. & Nesbitt, A. Dissociative chemisorption of O2 on Cu(110). Surf. Sci. 293, 211–226 (1993).
Orozco, I. et al. In situ studies of methanol decomposition over Cu(111) and Cu2O/Cu(111): effects of reactant pressure, surface morphology, and hot spots of active sites. J. Phys. Chem. C. 125, 558–571 (2021).
Seo, W.-W., Yim, J.-H., Kang, J. W. & Lim, J. S. Isobaric vapor–liquid equilibrium data of binary mixtures of [water + 2,3-butanediol] and [water + 1,4-butanediol] at 40, 50, 60, 66.7, 80, and 101 kPa. Korean J. Chem. Eng. 41, 1457–1466 (2024).
Xu, G. & Wang, Q. Chemically recyclable polymer materials: polymerization and depolymerization cycles. Green. Chem. 24, 2321–2346 (2022).
Liu, Y., Wu, J., Hu, X., Zhu, N. & Guo, K. Advances, Challenges, and Opportunities of Poly(γ-butyrolactone)-Based Recyclable Polymers. ACS Macro Lett. 10, 284–296 (2021).
Cargill to build biobased 1,4-butanediol plant. CEN Glob. Enterp. 99, 10–10 (2021).
Mutel, C. Brightway: an open source framework for life cycle assessment. J. Open Source Softw. 2, 236 (2017).
Steubing, B., De Koning, D., Haas, A. & Mutel, C. L. The activity browser—an open source LCA software building on top of the brightway framework. Softw. Impacts 3, 100012 (2020).
Huijbregts, M. A. et al. ReCiPe2016: a harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life cycle Assess. 22, 138–147 (2017).
Wernet, G. et al. The ecoinvent database version 3 (part I): overview and methodology. Int. J. Life Cycle Assess. 21, 1218–1230 (2016).
Mokwatlo, S. C. et al. Bioprocess development and scale-up for cis,cis-muconic acid production from glucose and xylose by Pseudomonas putida. Green. Chem. 26, 10152–10167 (2024).
Uekert, T. et al. Life cycle assessment of enzymatic poly(ethylene terephthalate) recycling. Green. Chem. 24, 6531–6543 (2022).
Padanyi, Z. V. The Raman spectrum of Ca(OH)2. Solid State Commun. 8, 541–543 (1970).
Balachandran, U. & Eror, N. G. Laser-induced Raman scattering in calcium titanate. Solid State Commun. 44, 815–818 (1982).
Lu, B. et al. Spatially resolved product speciation during struvite synthesis from magnesite (MgCO3) particles in ammonium (NH4+) and phosphate (PO43–) aqueous solutions. J. Phys. Chem. C. 123, 8908–8922 (2019).
Xu, J. F. et al. Raman spectra of CuO nanocrystals. J. Raman Spectrosc. 30, 413–415 (1999).
Deng, Y., Handoko, A. D., Du, Y., Xi, S. & Yeo, B. S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: identification of cuiii oxides as catalytically active species. ACS Catal. 6, 2473–2481 (2016).
Luo, M.-F., Fang, P., He, M. & Xie, Y.-L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A Chem. 239, 243–248 (2005).
Xia, Y., Yuan, P., Zhang, Y., Sun, Y. & Hong, M. Converting non-strained γ-valerolactone and derivatives into sustainable polythioesters via isomerization-driven cationic ring-opening polymerization of thionolactone intermediate. Angew. Chem. Int. Ed. 62, e202217812 (2023).
Yuan, P. et al. Library of stereoregular polythioesters for stereocomplex formation enabled by isomerization-driven cationic ring-opening polymerization. Angew. Chem. Int. Ed. e202501485, https://doi.org/10.1002/anie.202501485.
Li, X.-L., Clarke, R. W., Jiang, J.-Y., Xu, T.-Q. & Chen, E. Y. X. A circular polyester platform based on simple gem-disubstituted valerolactones. Nat. Chem. 15, 278–285 (2023).
Schneiderman, D. K. et al. Chemically recyclable biobased polyurethanes. ACS Macro Lett. 5, 515–518 (2016).
Brutman, J. P., De Hoe, G. X., Schneiderman, D. K., Le, T. N. & Hillmyer, M. A. Renewable, degradable, and chemically recyclable cross-linked elastomers. Ind. Eng. Chem. Res. 55, 11097–11106 (2016).
Ho, J. et al. Regional Energy Deployment System (reeds) Model Documentation (version 2020) (National Renewable Energy Lab.(NREL), Golden, CO (United States), 2021).
Acknowledgements
Funding was provided by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Materials and Manufacturing Technologies Office (AMMTO), and Bioenergy Technologies Office (BETO). This work was performed as part of the BioOptimized Technologies to keep Thermoplastics out of Landfills and the Environment (BOTTLE) Consortium and was supported by AMMTO and BETO at the National Renewable Energy Laboratory for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. The BOTTLE Consortium includes members from SLAC. D.K. was in part supported by the Director’s Fellowship - Laboratory Directed Research and Development (LDRD) Program at NREL. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes. We thank Professor Eugene Chen (CSU) and Dr. Tobias Hull (NREL) for their insightful comments and discussion.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.K., G.R., G.T.B.; methodology, D.K., G.R., F.I., O.B., J.S.D., A.P., E.V.R.; investigation, D.K., G.R., O.B., F.I., J.S.D., A.P. E.V.R.; writing—original draft, D.K., G.R., O.B., F.I., J.S.D., A.P., E.V.R.; writing—review & editing, D.K., G.R., O.B., F.I., J.S.D., A.P., E.V.R., G.T.B., I.H., S.R.B.; funding acquisition, G.T.B., I.H., S.R.B.; resources G.T.B., I.H., S.R.B.; supervision, G.T.B., I.H., S.R.B.
Corresponding authors
Ethics declarations
Competing interests
D.K., G.R., and G.T.B. have filed a provisional patent application related to this work, No.63/790,923. Other authors have no competing interests to declare.
Peer review
Peer review information
Nature Communications thanks Saikat Dutta, Bhari Mallanna Nagaraja, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kiani, D., Rosetto, G., Ibrahim, F. et al. Solventless, ambient-pressure production of bio-based lactones over earth-abundant, mixed metal oxide catalysts for circular polyesters. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69362-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69362-8