Abstract
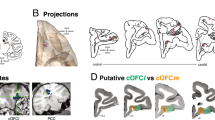
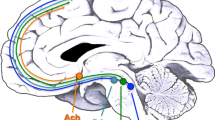
Mood disorders are associated with complex disruptions in brain networks, including those associated with the orbitofrontal cortex (OFC) and pregenual anterior cingulate cortex (pACC). Differential functions of these regions, especially the functions of the far-caudal OFC, are incompletely understood. We trained macaques to perform an approach-avoidance task and recorded cOFC and pACC neuronal activity and autonomic/somatic responses during performance, including during electrical microstimulation (EMS) of the cOFC. The cOFC was sensitive to both positive and negative stimuli, whereas the pACC was significantly more active during aversive outcomes. cOFC EMS increased avoidance, suggesting a causal cOFC function in cost-benefit decision-making. The cOFC activity led pACC activity during the decision period, supporting cOFC network prominence. Autonomic and somatic responses were positively correlated with behavioral patterns, consistent with a coordinated body-brain involvement during emotionally significant decision-making. We suggest that dysfunction of this network could potentially contribute to the etiology of mood disorders.
Similar content being viewed by others
Data availability
The Source Data underlying all main figures and Supplementary Figs. have been deposited in Figshare under the accession code doi: 10.6084/m9.figshare.30652049. Sample datasets used to illustrate the analysis workflow are provided in the associated GitHub repository. Other data that cannot be formatted in Excel (including large raw continuous electrophysiological and physiological recordings and related files in specialized formats that are not practical to deposit as Excel-compatible tables) are available under restricted access and may be requested by contacting the corresponding authors at: graybiel@mit.edu and georgios.k.papageorgiou@gmail.com.
Code availability
All MATLAB code used for data analyses and figure generation is available at: https://github.com/geokpap/natcomm_gp. The repository includes all scripts and a README file with instructions for reproducing the workflow using the accompanying sample datasets.
References
Mayberg, H. S. Limbic-cortical dysregulation: a proposed model of depression. https://doi.org/10.1176/jnp.9.3.471 (1997).
Drevets, W. C., Price, J. L. & Furey, M. L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. https://doi.org/10.1007/s00429-008-0189-x (2008).
Price, J. L. & Drevets, W. C. Neurocircuitry of mood disorders. https://doi.org/10.1038/npp.2009.104 (2010).
Ressler, K. J. & Mayberg, H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. https://doi.org/10.1038/nn1944 (2007).
Xu, X. et al. Intrinsic connectivity of the prefrontal cortex and striato-limbic system respectively differentiate major depressive from generalized anxiety disorder. https://doi.org/10.1038/S41386-020-00868-5 (2021).
Chrysikou, E. G., Wing, E. K. & van Dam, W. O. Transcranial Direct Current Stimulation Over the Prefrontal Cortex in Depression Modulates Cortical Excitability in Emotion Regulation Regions as Measured by Concurrent Functional Magnetic Resonance Imaging: An Exploratory Study. https://doi.org/10.1016/j.bpsc.2019.12.004 (2022).
Volkow, N. D., Wang, G. J., Fowler, J. S. & Tomasi, D. Addiction circuitry in the human brain. https://doi.org/10.1146/annurev-pharmtox-010611-134625 (2012).
Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. https://doi.org/10.1016/j.tics.2010.11.004 (2011).
Drevets, W. C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. https://doi.org/10.1038/386824a0 (1997).
Mayberg, H. S. et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. https://doi.org/10.1176/ajp.156.5.675 (1999).
Rushworth, M. F. & Behrens, T. E. Choice, uncertainty and value in prefrontal and cingulate cortex. https://doi.org/10.1038/nn2066 (2008).
Rushworth, M. F., Noonan, M. P., Boorman, E. D., Walton, M. E. & Behrens, T. E. Frontal cortex and reward-guided learning and decision-making. https://doi.org/10.1016/j.neuron.2011.05.014 (2011).
Kennerley, S. W., Walton, M. E., Behrens, T. E., Buckley, M. J. & Rushworth, M. F. Optimal decision making and the anterior cingulate cortex. https://doi.org/10.1038/nn1724 (2006).
Shenhav, A., Botvinick, M. M. & Cohen, J. D. The expected value of control: an integrative theory of anterior cingulate cortex function. https://doi.org/10.1016/j.neuron.2013.07.007 (2013).
Amemori, K., Amemori, S. & Graybiel, A. M. Motivation and affective judgments differentially recruit neurons in the primate dorsolateral prefrontal and anterior cingulate cortex. https://doi.org/10.1523/JNEUROSCI.1731-14.2015 (2015).
Amemori, K. & Graybiel, A. M. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat. Neurosci. 15, 776–785 (2012).
Amemori, S., Graybiel, A. M. & Amemori, K. I. Causal Evidence for Induction of Pessimistic Decision-Making in Primates by the Network of Frontal Cortex and Striosomes. https://doi.org/10.3389/fnins.2021.649167 (2021).
Ironside, M. et al. Approach-Avoidance Conflict in Major Depressive Disorder: Congruent Neural Findings in Humans and Nonhuman Primates. https://doi.org/10.1016/j.biopsych.2019.08.022 (2020).
Pizzagalli, D. A. & Roberts, A. C. Prefrontal cortex and depression. https://doi.org/10.1038/s41386-021-01101-7 (2022).
Kringelbach, M. L. & Rolls, E. T. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. https://doi.org/10.1016/j.pneurobio.2004.03.006 (2004).
Schoenbaum, G., Setlow, B., Saddoris, M. P. & Gallagher, M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. https://doi.org/10.1016/s0896-6273(03 (2003).
Padoa-Schioppa, C. & Assad, J. A. Neurons in the orbitofrontal cortex encode economic value. https://doi.org/10.1038/nature04676 (2006).
O’Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J. & Andrews, C. Abstract reward and punishment representations in the human orbitofrontal cortex. https://doi.org/10.1038/82959. (2001).
Chau, B. K. et al. Contrasting roles for orbitofrontal cortex and amygdala in credit assignment and learning in macaques. https://doi.org/10.1016/j.neuron.2015.08.018 (2015).
Papageorgiou, G. K. et al. Inverted activity patterns in ventromedial prefrontal cortex during value-guided decision-making in a less-is-more task. https://doi.org/10.1038/s41467-017-01833-5 (2017).
Kahnt, T., Heinzle, J., Park, S. Q. & Haynes, J. D. The neural code of reward anticipation in human orbitofrontal cortex. https://doi.org/10.1073/pnas.0912838107 (2010).
Ballesta, S., Shi, W., Conen, K. E. & Padoa-Schioppa, C. Values encoded in orbitofrontal cortex are causally related to economic choices. https://doi.org/10.1038/S41586-020-2880-x (2020).
Rudebeck, P. H. & Murray, E. A. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. https://doi.org/10.1016/j.neuron.2014.10.049 (2014).
Murray, E. A., Moylan, E. J., Saleem, K. S., Basile, B. M. & Turchi, J. Specialized areas for value updating and goal selection in the primate orbitofrontal cortex. https://doi.org/10.7554/eLife.11695 (2015).
Barbas, H. & Pandya, D. N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. https://doi.org/10.1002/cne.902860306 (1989).
Carmichael, S. T. & Price, J. L. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J. Comp. Neurol. 346, 366–402 (1994).
Schmahmann, J. D. & Pandya, D. N. Fiber Pathways of the Brain. https://doi.org/10.1093/acprof:oso/9780195104233.001.0001 (2006).
Barbas, H. & Rempel-Clower, N. Cortical structure predicts the pattern of corticocortical connections. https://doi.org/10.1093/cercor/7.7.635 (1997).
Ghashghaei, H. T., Hilgetag, C. C. & Barbas, H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. https://doi.org/10.1016/j.neuroimage.2006.09.046 (2007).
Eblen, F. & Graybiel, A. M. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. https://doi.org/10.1523/JNEUROSCI.15-09-05999.1995 (1995).
Amemori, S. et al. Microstimulation of primate neocortex targeting striosomes induces negative decision-making. Eur. J. Neurosci. 51, 731–741 (2020).
Murray, E. A. & Fellows, L. K. Prefrontal cortex interactions with the amygdala in primates. https://doi.org/10.1038/S41386-021-01128-w (2022).
Amemori, S., Graybiel, A. M. & Amemori, K. I. Cingulate microstimulation induces negative decision-making via reduced top-down influence on primate fronto-cingulo-striatal network. https://doi.org/10.1038/S41467-024-48375-1 (2024).
Amemori, K. & Graybiel, A. M. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. https://doi.org/10.1038/nn.3088 (2012).
Amemori, K. I., Amemori, S., Gibson, D. J. & Graybiel, A. M. Striatal Microstimulation Induces Persistent and Repetitive Negative Decision-Making Predicted by Striatal Beta-Band Oscillation. https://doi.org/10.1016/j.neuron.2018.07.022 (2018).
Millan, M. J. The neurobiology and control of anxious states. https://doi.org/10.1016/S0301-0082(03)00087-X (2003).
Aupperle, R. L., Melrose, A. J., Francisco, A., Paulus, M. P. & Stein, M. B. Neural substrates of approach-avoidance conflict decision-making. https://doi.org/10.1002/hbm.22639 (2015).
Friedman, A. et al. A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict. https://doi.org/10.1016/j.cell.2015.04.049 (2015).
Friedman, A. et al. Chronic Stress Alters Striosome-Circuit Dynamics, Leading to Aberrant Decision-Making. https://doi.org/10.1016/j.cell.2017.10.017 (2017).
Friedman, A. et al. Striosomes Mediate Value-Based Learning Vulnerable in Age and a Huntington’s Disease Model. https://doi.org/10.1016/j.cell.2020.09.060 (2020).
Papageorgiou, G. K., Baudonnat, M., Cucca, F. & Walton, M. E. Mesolimbic dopamine encodes prediction errors in a state-dependent manner. https://doi.org/10.1016/j.celrep.2016.03.031 (2016).
McFadden, D. Conditional Logit Analysis of Qualitative Choice Behavior. In Frontiers in Econometrics (ed. Zarembka, P.) 105–142 (Academic Press, New York) (1974).
Levy, I., Snell, J., Nelson, A. J., Rustichini, A. & Glimcher, P. W. Neural representation of subjective value under risk and ambiguity. https://doi.org/10.1152/jn.00853.2009 (2010).
Von Neumann, J. & Morgenstern, O. Theory of Games and Economic Behavior 2nd rev. edn. (Princeton Univ. Press, Princeton, NJ) (1947).
Padoa-Schioppa, C. Neurobiology of economic choice: a good-based model. https://doi.org/10.1146/annurev-neuro-061010-113648 (2011).
Mnih, V. et al. Asynchronous methods for deep reinforcement learning. In Proc. 33rd International Conference on Machine Learning. Proc. Mach. Learn. Res. (eds Balcan, M. F. & Weinberger, K. Q.) Vol. 48, 1928–1937 (PMLR, 2016).
Johnson, E. J. & Ratcliff, R. Computational and process models of decision making in psychology and behavioral economics. In Neuroeconomics: Decision Making and the Brain 2nd edn. (eds Glimcher, P. W. & Fehr, E.) 35–47 (Academic Press, 2014). https://doi.org/10.1016/B978-0-12-416008-8.00003-6
Wallis, J. D. Orbitofrontal cortex and its contribution to decision-making. https://doi.org/10.1146/annurev.neuro.30.051606.094334 (2007).
Rolls, E. T. The functions of the orbitofrontal cortex. https://doi.org/10.1016/S0278-2626(03 (2004).
Shackman, A. J. et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. https://doi.org/10.1038/nrn2994 (2011).
Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., 3rd, & Wager, T. D. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. https://doi.org/10.1016/j.neubiorev.2011.11.009 (2012).
Appelhans, B. M. & Luecken, L. J. Heart rate variability as an index of regulated emotional responding. https://doi.org/10.1037/1089-2680.10.3.229 (2006).
Thayer, J. F. & Lane, R. D. A model of neurovisceral integration in emotion regulation and dysregulation. https://doi.org/10.1016/s0165-0327(00 (2000).
Bradley, M. M., Miccoli, L., Escrig, M. A. & Lang, P. J. The pupil as a measure of emotional arousal and autonomic activation. https://doi.org/10.1111/j.1469-8986.2008.00654.x (2008).
Nassar, M. R. et al. Rational regulation of learning dynamics by pupil-linked arousal systems. https://doi.org/10.1038/nn.3130 (2012).
Murphy, P. R., Vandekerckhove, J. & Nieuwenhuis, S. Pupil-linked arousal determines variability in perceptual decision making. https://doi.org/10.1371/journal.pcbi.1003854 (2014).
Kalin, N. H., Shelton, S. E. & Davidson, R. J. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. https://doi.org/10.1111/j.1467-8624.1991.tb01598.x (1991).
Kalin, N. H., Shelton, S. E. & Davidson, R. J. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. https://doi.org/10.1037/0735-7044.112.1.251 (1996).
Sammito, S. & Thielmann, B. The circadian rhythm of heart rate variability. https://doi.org/10.1080/09291016.2016.1183887 (2020).
Folkedal, O. et al. Food anticipatory behaviour as an indicator of stress response and recovery in Atlantic salmon post-smolt after exposure to acute temperature fluctuation. https://doi.org/10.1016/j.physbeh.2011.08.008 (2012).
Gygax, L., Reefmann, N., Wolf, M. & Langbein, J. Prefrontal cortex activity, sympatho-vagal reaction and behaviour distinguish between situations of feed reward and frustration in dwarf goats. https://doi.org/10.1016/j.bbr.2012.10.052 (2013).
Feingold, J. et al. A system for recording neural activity chronically and simultaneously from multiple cortical and subcortical regions in nonhuman primates. https://doi.org/10.1152/jn.00625.2011 (2012).
Hwang, J., Mitz, A. R., & Murray, E. A. NIMH MonkeyLogic: Behavioral control and data acquisition in MATLAB. https://doi.org/10.1016/j.jneumeth.2019.05.002 (2019).
Acknowledgements
We thank H. F. Hall, and Y. Kubota (Massachusetts Institute of Technology) for technical support, research insight, and manuscript preparation. This research was supported by the National Institute of Mental Health (P50 MH119467, to A.M.G.), the National Institute of Neurological Disorders and Stroke (R01 NS025529, to A.M.G.), the Army Research Office (W911NF-16-1-0474, to A.M.G.), the Japan Society for the Promotion of Science (JP24H02163 and JP22H04998, to K.A.), the Japan Agency for Medical Research and Development (JP24wm0625210h and JP24gm6910012h, to K.A.), the K. Lisa Yang Integrative Computational Neuroscience Center (to R.G.Y.) and the Mercatus Center at George Mason University (Emergent Ventures fellowship, to M.C.W.).
Author information
Authors and Affiliations
Contributions
Conceptualization, G.K.P., K.A., and A.M.G.; Methodology, G.K.P., K.A., and A.M.G.; Experimental Software, G.K.P.; Formal Analysis, G.K.P., D.J.G., K.A., M.N., and G.R.Y.; Writing—Original Draft, G.K.P.; Review, D.J.G., A.M.G., K.A., and H.N.S.; Experimental work, G.K.P., K.A., H.N.S., M.C.W., J.S., U.U., T.Y., and A.M.G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks James Howard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Papageorgiou, G.K., Amemori, Ki., Gibson, D.J. et al. Functional distinctions between orbitofrontal cortex and anterior cingulate cortex subregions in decision-making and autonomic regulation. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69447-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69447-4