Abstract
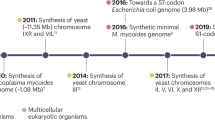
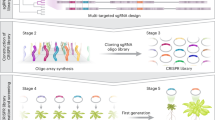
The de novo synthesis of genomes has made unprecedented progress and achieved milestones, particularly in bacteria and yeast. However, the process of synthesizing a multicellular plant genome has not progressed at the same pace, due to the complexity of multicellular plant genomes, technical difficulties associated with large genome size and structure, and the intricacies of gene regulation and expression in plants. Here we outline the bottom-up design principles for the de novo synthesis of the Physcomitrium patens (that is, earthmoss) genome. To facilitate international collaboration and accessibility, we have developed and launched a public online design platform called GenoDesigner. This platform offers an intuitive graphical interface enabling users to efficiently manipulate extensive genome sequences, even up to the gigabase level. This tool is poised to greatly expedite the synthesis of the P. patens genome, offering an essential reference and roadmap for the synthesis of plant genomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All genome data used in the design of SynMoss are available via Figshare at https://doi.org/10.6084/m9.figshare.22975925.v5 (ref. 51).
Code availability
The source code and instructions for GenoDesigner are available at https://github.com/SynMoss/GenoDesigner. The server is accessible at http://49.51.34.198:3000/client/chromosome/en/#/. Related Python and R programs are available at https://github.com/WenfeiY/P.patens_geno_design.
Change history
07 June 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41477-024-01734-8
References
Sinyakov, A. N., Ryabinin, V. A. & Kostina, E. V. Application of array-based oligonucleotides for synthesis of genetic designs. Mol. Biol. (Mosk.) 55, 562–577 (2021).
He, B. et al. YLC-assembly: large DNA assembly via yeast life cycle. Nucleic Acids Res. 51, 8283–8292 (2023).
Hanna, R. E. & Doench, J. G. Design and analysis of CRISPR–Cas experiments. Nat. Biotechnol. 38, 813–823 (2020).
Gibson, D. G. et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 319, 1215–1220 (2008).
Dymond, J. S. et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 477, 471–476 (2011).
Fredens, J. et al. Total synthesis of Escherichia coli with a recoded genome. Nature 569, 514–518 (2019).
Cello, J., Paul, A. V. & Wimmer, E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science 297, 1016–1018 (2002).
Smith, H. O., Hutchison, C. A. 3rd, Pfannkoch, C. & Venter, J. C. Generating a synthetic genome by whole genome assembly: phiX174 bacteriophage from synthetic oligonucleotides. Proc. Natl Acad. Sci. USA 100, 15440–15445 (2003).
Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56 (2010).
Annaluru, N. et al. Total synthesis of a functional designer eukaryotic chromosome. Science 344, 55–58 (2014).
Mitchell, L. A. et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond. Science https://doi.org/10.1126/science.aaf4831 (2017).
Shen, Y. et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome. Science https://doi.org/10.1126/science.aaf4791 (2017).
Vashee, S. et al. Cloning, assembly, and modification of the primary human cytomegalovirus isolate toledo by yeast-based transformation-associated recombination. mSphere 2, e00331–00317 (2017).
Wu, Y. et al. Bug mapping and fitness testing of chemically synthesized chromosome X. Science https://doi.org/10.1126/science.aaf4706 (2017).
Xie, Z. X. et al. ‘Perfect’ designer chromosome V and behavior of a ring derivative. Science https://doi.org/10.1126/science.aaf4704 (2017).
Blount, B. A. et al. Synthetic yeast chromosome XI design provides a testbed for the study of extrachromosomal circular DNA dynamics. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100418 (2023).
Foo, J. L. et al. Establishing chromosomal design–build–test–learn through a synthetic chromosome and its combinatorial reconfiguration. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100435 (2023).
Lauer, S. et al. Context-dependent neocentromere activity in synthetic yeast chromosome VIII. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100437 (2023).
Luo, J. et al. Synthetic chromosome fusion: effects on mitotic and meiotic genome structure and function. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100439 (2023).
McCulloch, L. H. et al. Consequences of a telomerase-related fitness defect and chromosome substitution technology in yeast synIX strains. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100419 (2023).
Schindler, D. et al. Design, construction, and functional characterization of a tRNA neochromosome in yeast. Cell https://doi.org/10.1016/j.cell.2023.10.015 (2023).
Shen, Y. et al. Dissecting aneuploidy phenotypes by constructing Sc2.0 chromosome VII and SCRaMbLEing synthetic disomic yeast. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100364 (2023).
Williams, T. C. et al. Parallel laboratory evolution and rational debugging reveal genomic plasticity to S. cerevisiae synthetic chromosome XIV defects. Cell Genom. https://doi.org/10.1016/j.xgen.2023.100379 (2023).
Zhang, W. et al. Manipulating the 3D organization of the largest synthetic yeast chromosome. Mol. Cell 83, 4424–4437.e5 (2023).
Zhao, Y. et al. Debugging and consolidating multiple synthetic chromosomes reveals combinatorial genetic interactions. Cell 186, 5220–5236.e16 (2023).
Boeke, J. D. et al. The Genome Project-Write. Science 353, 126–127 (2016).
Dai, J., Boeke, J. D., Luo, Z., Jiang, S. & Cai, Y. Sc3.0: revamping and minimizing the yeast genome. Genome Biol. 21, 205 (2020).
Rensing, S. A., Goffinet, B., Meyberg, R., Wu, S.-Z. & Bezanilla, M. The moss Physcomitrium (Physcomitrella) patens: a model organism for non-seed plants. Plant Cell 32, 1361–1376 (2020).
Lang, D. et al. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 93, 515–533 (2018).
Bi, G. et al. Near telomere-to-telomere genome of the model plant Physcomitrium patens. Nat. Plants 10, 327–343 (2024).
Ye, Z.-H. & Zhong, R. Cell wall biology of the moss Physcomitrium patens. J. Exp. Bot. 73, 4440–4453 (2022).
Chen, L.-G. et al. A designer synthetic chromosome fragment functions in moss. Nat. Plants 10, 228–239 (2024).
Richardson, S. M. et al. Design of a synthetic yeast genome. Science 355, 1040–1044 (2017).
Liu, W. & Stewart, C. N. Plant synthetic promoters and transcription factors. Curr. Opin. Biotechnol. 37, 36–44 (2016).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Ostrov, N. et al. Design, synthesis, and testing toward a 57-codon genome. Science 353, 819–822 (2016).
Robertson, W. E. et al. Sense codon reassignment enables viral resistance and encoded polymer synthesis. Science 372, 1057–1062 (2021).
Venter, J. C., Glass, J. I., Hutchison, C. A. & Vashee, S. Synthetic chromosomes, genomes, viruses, and cells. Cell 185, 2708–2724 (2022).
Dymond, J. & Boeke, J. The Saccharomyces cerevisiae SCRaMbLE system and genome minimization. Bioeng. Bugs 3, 168–171 (2012).
Shen, Y. et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes. Genome Res. 26, 36–49 (2016).
Mercy, G. et al. 3D organization of synthetic and scrambled chromosomes. Science https://doi.org/10.1126/science.aaf4597 (2017).
Blount, B. A. et al. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome. Nat. Commun. 9, 1932 (2018).
Jia, B. et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat. Commun. https://doi.org/10.1038/s41467-018-03084-4 (2018).
Anastasiadou, E., Jacob, L. S. & Slack, F. J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 18, 5–18 (2018).
Kobayashi, W. et al. Homologous pairing activities of Arabidopsis thaliana RAD51 and DMC1. J. Biochem. 165, 289–295 (2019).
Kamisugi, Y. et al. The mechanism of gene targeting in Physcomitrella patens: homologous recombination, concatenation and multiple integration. Nucleic Acids Res. 34, 6205–6214 (2006).
Galdzicki, M., Rodriguez, C., Chandran, D., Sauro, H. M. & Gennari, J. H. Standard Biological Parts Knowledgebase. PLoS ONE 6, e17005 (2011).
Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014).
Wright, E. S. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J. 8, 352–359 (2016).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Zhao, S. Telomere-to-telomere (T2T) genome of the model plant Physcomitrium patens. Figshare https://doi.org/10.6084/m9.figshare.22975925.v5 (2023).
Acknowledgements
This study was supported by the National Key R&D Program of China (grant nos 2022YFF1201800, 2021YFF1201700 and 2019YFA0903900), the National Natural Science Foundation of China (grant no. 32201207), the Innovation Program of the Chinese Academy of Agricultural Sciences, the Shenzhen Science and Technology Program (grant nos KQTD20180413181837372 and RCYX20221008092950122), the Shenzhen Outstanding Talents Training Fund, the CAS Project for Young Scientists in Basic Research (grant no. YSBR-078) and the Strategic Priority Research Program (Precision Seed Design and Breeding, grant no. XDA24020103).
Author information
Authors and Affiliations
Contributions
J.Y., Q.Z., B.M., Y.W., Y.J., Y.M., X.H., W.Q. and J.D. conceived and designed the project. X.H., W.Q. and J.D. supervised the research. W.Y., S. Zhang and X.H. completed the programming. S. Zhao analysed the RNA-seq data. L.-g.C., J.C. and H.Y. performed the experiments. W.Y., S. Zhang, X.H., W.Q. and J.D. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, W., Zhang, S., Zhao, S. et al. Designing a synthetic moss genome using GenoDesigner. Nat. Plants 10, 848–856 (2024). https://doi.org/10.1038/s41477-024-01693-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41477-024-01693-0
This article is cited by
-
Genome synthesis in plants
Nature Reviews Bioengineering (2025)
-
The design and engineering of synthetic genomes
Nature Reviews Genetics (2025)