Abstract
A multi-subunit enzyme, cytochrome b6f (cytb6f), provides the crucial link between photosystems I and II in the photosynthetic membranes of higher plants, transferring electrons between plastoquinone (PQ) and plastocyanin. The atomic structure of cytb6f is known, but its detailed catalytic mechanism remains elusive. Here we present cryogenic electron microscopy structures of spinach cytb6f at 1.9 Å and 2.2 Å resolution, revealing an unexpected orientation of the substrate PQ in the haem ligand niche that forms the PQ reduction site (Qn). PQ, unlike Qn inhibitors, is not in direct contact with the haem. Instead, a water molecule is coordinated by one of the carbonyl groups of PQ and can act as the immediate proton donor for PQ. In addition, we identify water channels that connect Qn with the aqueous exterior of the enzyme, suggesting that the binding of PQ in Qn displaces water through these channels. The structures confirm large movements of the head domain of the iron–sulfur protein (ISP-HD) towards and away from the plastoquinol oxidation site (Qp) and define the unique position of ISP-HD when a Qp inhibitor (2,5-dibromo-3-methyl-6-isopropylbenzoquinone) is bound. This work identifies key conformational states of cytb6f, highlights fundamental differences between substrates and inhibitors and proposes a quinone–water exchange mechanism.
Similar content being viewed by others
Main
Photosynthesis powers the metabolism of photoautotrophic organisms by converting solar energy into biologically useful forms of energy. In higher plants, photosynthesis is accomplished in the thylakoid membranes of chloroplasts by electron transport chains composed of two large membrane protein complexes—photosystems I and II—that are functionally linked by cytochrome b6f (cytb6f)1,2,3. By catalyzing the transfer of electrons from lipid-soluble plastoquinone (PQ) to water-soluble plastocyanin (PC), cytb6f transfers electrons from photosystem II to photosystem I. This results in proton transfer across the membrane and contributes to the proton-motive force required for the production of adenosine triphosphate4. Cytb6f is also involved in regulatory processes that secure photosynthetic efficiency5,6,7,8. Cytb6f is, thus, a crucial enzyme for photosynthesis. Yet, some of the most fundamental aspects of its mechanism remain unclear.
Cytb6f operates according to a modified ‘Q-cycle’ that was originally developed for cytochrome bc1 (cytbc1)4,9. Cytbc1 is related to cytb6f and performs a similar function in the respiratory or photosynthetic electron transport chains. By analogy to cytbc1, the cycle in cytb6f is based on the joint operation of two catalytic sites, Qp and Qn. These sites are located on opposite sides of the membrane and are responsible for plastoquinol (PQH2) oxidation (at the Qp site, on the luminal side of the membrane) and PQ reduction (at the Qn site, on the stromal side)10. These sites are connected functionally by redox cofactors organized in two chains: a ‘low-potential’ chain comprising b-type haems bp and bn and c-type haem cn (also referred to as haem ci), and a ‘high-potential’ chain comprising an iron–sulfur cluster [2Fe2S] and a c-type haem f11. The Qp acts as a branch point for both chains, where it is linked to Qn in the low-potential chain and to PC in the high-potential chain (Fig. 1a). The oxidation of PQH2 at Qp separates the routes for two electrons deriving from this reaction. One electron is transferred to PC via the [2Fe2S] and haem f, and the other is transferred via haem bp and the pair of haems bn/cn to ultimately reduce quinone at Qn. As a consequence of the bifurcation reaction, the low-potential chain delivers one electron to Qn at a time. Thus, the oxidation of two PQH2 at Qp leads to reduction of one PQ at Qn, which results in a net oxidation of PQH2 (refs. 3,6).
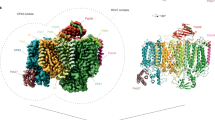
a, Arrangement of redox cofactor chains in cytb6f and a schematic representation of PQH2 oxidation and PQ reduction at Qp and Qn, respectively. Green-filled boxes represent two monomers of cytb6f. PC stands for plastocyanin. b, Two procedures for cryo-EM grid preparation, which yielded 1.9 Å and 2.2 Å cryo-EM maps for DPQ-incubated cytb6f and cytb6f during enzymatic turnover, respectively. c, Cryo-EM maps at 1.9 Å resolution of DPQ-incubated cytb6f with colour-coded subunits (left) and coloured according to the calculated local resolution (in Å; right). d, Close-up view of selected 1.9 Å map regions with remarkable resolution of amino acid side chains and cofactors. The colours of the frames correspond to regions marked by squares in c.
Various structures of cytbc1 and cytb6f show that the positions of cofactors are consistent with a functional Q-cycle in both complexes12,13,14,15,16,17,18,19,20,21,22. Nevertheless, intriguing differences between cytbc1 and cytb6f also indicate functional differences. For instance, the cytb6f complex contains additional cofactors—chlorophyll a, 9-cis β-carotene and haem cn—whose functions are unclear. Haem cn is highly unusual. It is a high-spin haem with a water molecule as the only axial ligand to the haem iron. It is covalently attached to a single cysteine residue and positioned in proximity to haem bn (refs. 15,16,20). Moreover, it occupies the position where ubiquinone binding occurs in cytbc1. Haem cn thus appears to be an extension of the low-potential chain (Fig. 1a), but the mechanistic consequences of this arrangement remain highly speculative. Inhibitors of Qn have been shown to bind as axial ligands to haem cn, and it has been postulated that the substrate binds in a similar way17,18,23. However, the reported positions of PQ in the region of Qn are inconsistent with the overall model and, thus, have been considered precatalytic states22,24.
Currently available structures of cytb6f show a large distance between Qp and haem f, similar to the large distance between Qp and haem c1 (equivalent of haem f) observed in cytbc1. This implies that large-scale movement of the domain harbouring the iron–sulfur cluster (ISP-HD) between positions close to Qp and haem f/c1 must occur during the catalytic cycle25. Indeed, such movement is well documented for cytbc1, not only by numerous spectroscopic and kinetic analyses26,27,28,29 but also by structural data showing ISP-HD in various positions30. In the case of cytb6f, spectroscopic data suggest a similar movement of ISP-HD31,32,33; however, distinct positions of ISP-HD have not yet been structurally characterized.
To simultaneously address these open questions, we determined high-resolution, single-particle cryogenic electron microscopy (cryo-EM) structures of spinach cytb6f in various states (Fig. 1b). We show that decylplastoquinone (DPQ), an analogue of the natural PQ substrate, adopts an unprecedented position and orientation in the Qn site during catalysis. The observed coordination is fundamentally different from the known position of Qn site-specific inhibitors17,18,23. Our structures also reveal coordinated water channels that connect the exterior of the complex directly with Qn. Furthermore, we show that ISP-HD adopts distinct positions, which illustrate its dynamic movement between cyt b6 and cyt f. These findings provide fundamental molecular insights into the interactions at the catalytic sites of cytb6f that inspire new concepts for explaining substrate exchange in cytb6f and cytbc1 complexes.
Results
Cryo-EM structures of cytb 6 f with DPQ and during turnover
Purified and detergent-solubilized cytb6f was equilibrated with an excess of DPQ or exposed to substrates, reduced DPQ (DPQH2) and oxidized PC and vitrified during catalytic turnover. These samples were used for single-particle cryo-EM analyses and resulted in two high-resolution reconstructions. In detail, we determined the structure of cytb6f supplemented with DPQ at an overall resolution of 1.9 Å and the enzyme during catalysis at an overall resolution of 2.2 Å (Fig. 1b, Table 1 and Extended Data Fig. 1). Both structures show cytb6f as a homodimeric complex with a subunit composition and spatial arrangement of cofactors consistent with previous reports15,21,22 (Fig. 1c and Extended Data Fig. 1). The recently identified subunit thylakoid soluble phosphoprotein 9 (TSP9)21 is present in both structures, indicating that it is stably associated throughout the catalytic cycle. The quality of the cryo-EM maps obtained allowed us to unambiguously identify amino acid side chains, ligands and cofactors (Fig. 1d). Furthermore, we observed density peaks in various regions of cytb6f that likely correspond to coordinated water molecules, which we would expect to observe at 1.9 Å and 2.2 Å resolution. We assigned, validated and manually curated the positions of 329 water molecules in the 1.9 Å structure and 214 in the 2.2 Å structure.
DPQ does not contact the haem iron when bound at Qn
The 2.2 Å resolution structure of cytb6f vitrified during catalysis revealed the position and orientation of a quinone bound in Qn (Fig. 2a,b). Note that we use the term ‘quinone’ to indicate a quinone group, not its redox state, which cannot be inferred from the cryo-EM maps. In detail, the quinone head is inclined at an angle of 37° relative to the plane of haem cn, with the C4-side of the quinone ring positioned close to the haem plane (Fig. 2b,c). A water molecule (wat1) is coordinated between the carbonyl oxygen at the C4-side (O4) of quinone and the conserved Asp35 residue of subunit IV (D35sIV; Fig. 2b). D35sIV and wat1 are located inside a hydrophobic pocket formed by F40sIV, L36sIV, A31sIV and Val26 of the cytochrome b6 subunit (V26b6) near haem cn (Extended Data Fig. 2a). On the opposite side of the quinone group, the propionate of haem cn and R207b6 enclose the carbonyl oxygen (O1) of DPQ (Fig. 2b and Extended Data Fig. 2b). O1 is close enough to R207b6 and the proximal propionate to hydrogen bond with them. The distal propionate points towards a hydrophilic tunnel comprising K24b6, N25sIV and the backbone carbonyls of R207b6, K208b6 and Q209b6 (Extended Data Fig. 2b). The position of wat1 is remarkable because it suggests that this water molecule might be directly engaged in PQ reduction.
a, Quinone molecule (DPQ in all figures refers to a DPQ molecule, not its redox state) remodelled in the Qn site in the 2.2 Å cryo-EM map (PDB ID: 9ES8). b, Hydrogen-bond interactions between quinone, wat1, haem cn and selected residues. Cyt b6 (green), subIV (light teal) and haem bn (red) are in the background. c, Close-up side view of Qn with quinone and haem cn built into the cryo-EM map shown with isomesh representation. d, Close-up view of Qn occupied by the NQNO inhibitor bound to haem cn. The model and electron density map (that is, x-ray crystallographic 2Fo–Fc map) were taken from PDB ID: 4H0L entry17. e, Low-field regions of the EPR spectra of cytb6f poised at Eh of +180 mV or −20 mV, with (black) and without (red) addition of quinone. Black arrows indicate the regions with the largest observed changes, related to an increase in the rhombicity of the haem cn signal in the sample with quinone added. For comparison, the blue spectrum shows the relatively larger effect on the rhombicity of the haem cn signal imposed by interaction with NQNO bound at Qn.
The orientation of the quinone ring in Qn clearly differs from those of three Qn inhibitors described previously: 2-nonyl-4-hydroxyquinoline N-oxide (NQNO), tridecyl-stigmatellin and stigmatellin A. In currently known structures of cytb6f, these inhibitors coordinate the iron atom of haem cn directly17,18,23 (Fig. 2d and Extended Data Fig. 3). By analogy to these inhibitors, PQ was thought to bind to the haem cn in the same way. Our structure, however, clearly shows that although the quinone ring is in proximity to the haem, there are no direct contacts between the quinone and the haem iron (Fig. 2c). In fact, the phenyl group of F40sIV covers a large part of the haem plane, severely restricting access for the potential axial ligand15,34. We conclude that the inhibitors must push the F40sIV ring away upon binding to occupy the Qn niche and replace wat1.
The proximity of the quinone ring to haem cn was further substantiated by the sensitivity of the electron paramagnetic resonance (EPR) spectrum of this haem to the redox state of quinone (Fig. 2e), observed as changes in transitions at different g values (the g factor, abbreviated as g, characterizes magnetic moment and angular momentum of molecular system with unpaired electrons). At a high external redox potential (Eh = +180 mV), the spectrum showed a g = 12 transition that originates from the spin–spin exchange between the oxidized haems cn and bn (ref. 35) and the g = 6 signal, which can be ascribed to a fraction of nearly axial high-spin haem cn that is not spin-coupled to haem bn (ref. 36). This spectrum was insensitive to the presence of externally added DPQ (at this Eh, quinone remains oxidized). At a lower Eh (−20 mV), the signal at g = 12 disappeared37, but the remaining transitions around g = 6 were clearly sensitive to the presence of DPQH2 in the sample (at this Eh, the added DPQ becomes fully reduced). Changes in the shape of the lines around g = 6 suggest a slight increase in the rhombicity of the zero-field splitting36. Typically, an increase in rhombicity is recognized by splitting of the g = 6 line into two lines. In our case, the rhombicity is not large, and the split is small and results in overlapping transitions (arrows in Fig. 2e). Such spectral change is possibly a consequence of lowering the symmetry of the ligand field around the iron ion of haem cn, resulting from the presence of the quinone molecule nearby. However, we cannot conclude on the specific redox state of that molecule (that is, whether it is DPQH2 or the semiquinone form) involved in these interactions.
NQNO, which binds as a ligand to the iron of haem cn, induced different changes in the shape of the EPR spectrum (Fig. 2e, blue). Consistent with earlier observations36, even at high Eh, a dramatic increase in the rhombicity of the signal was accompanied by loss of the signal at g = 12. Thus, the EPR spectra not only confirm the proximity of the quinone or inhibitor to haem cn but also corroborate their different binding modes.
Proton channels and water molecules link Qn to the exterior
The 4 Å distance between haem cn and the quinone ring in the haem ligand niche confirms their readiness for the catalytically relevant electron transfer. Furthermore, hydrogen bonding indicates that the quinone is also ready to accept protons at O1 and O4, which are required for the completion of catalytic reduction of PQ (Figs. 2b and 3a). Wat1 is positioned to act as the primary proton donor for O4. Wat1 might accept a proton directly from D35sIV, which is surrounded by several polar residues (R26PetN, E29sIV, K272f (transmembrane helix of the cytochrome f subunit)), and several aligned water molecules extending towards the aqueous phase (Fig. 3a–d, red dots). On the other side of the ring, R207b6 is the most likely primary proton donor for O1; however, direct protonation by the proximal propionate of haem also appears possible. R207b6 might accept protons from D20b6, which is in direct contact with the protein exterior. Thus, these two residues might form an anhydrous proton channel towards O1, as proposed earlier17. R207b6 might also accept a proton from the proximal propionate of haem (Fig. 2b).
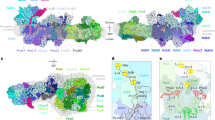
a, Schematic representation of water chains connecting the Qn cavity with the aqueous phase. The stromal and lumenal side of the thylakoid membrane are referred to as n-side and p-side, respectively. Red dots mark water molecules present in a funnel-like pocket in the cytb6f surface, in contact with wat1 through D35sIV. Purple dots represent water molecules reaching the distal propionate of haem cn. Blue dots represent a water molecule wire connected to R207b6. b, The 2.2 Å structure of Qn (PDB ID: 9ES8) with quinone bound, showing water molecules colour-coded the same as in a. c, The 1.9 Å structure of Qn (PDB ID: 9ES7) showing water molecules colour-coded the same as in a. d–f, Close-up view of each water chain (red (d), blue (e) and purple (f)) taken from the 1.9 Å structure with selected amino acids and isomesh representation of the cryo-EM map around water molecules. g, The proposed quinone/water exchange mechanism as a molecular basis for the efficiency of catalytic turnover. In this mechanism, the arrival or departure of PQ from Qn is synchronized with the movement of water molecules in and out of Qn (see details in the text). Wat1 and the two water molecules seen in the 1.9 Å structure that clash with quinone in the 2.2 Å structures are marked as red and blue dots, respectively.
In addition to the proton channels, our structures reveal channels filled with water molecules that line up, linking the Qn niche with the aqueous phase outside the membrane (Fig. 3). In one channel, water molecules (Fig. 3, blue dots) are located in the crevice between subunit IV and cytb6. They interact with the backbone atoms of A31sIV, W32sIV, T22b6, K24b6 and I21b6 (Fig. 3b,c,e). Two water molecules are positioned within hydrogen-bonding distance to the side chain of R207b6 (Fig. 3c). In another channel, the water molecules (Fig. 3, magenta dots) interact with the backbone atoms of G23sIV, Q209b6, R207b6 and the side chains of N25sIV and K24b6, reaching the distal propionate of haem cn (Fig. 3b,c,f). We note that the density map of the region of the Qn cavity in the 1.9 Å structure was not completely empty and may have been partially occupied by DPQ or endogenous quinone molecules. Nevertheless, two water molecules that are hydrogen-bonded by R207b6 can be clearly identified. When quinone occupies the catalytically relevant position (as seen in the 2.2 Å structure), these water molecules would clash with the quinone ring and, thus, must be displaced during the full accommodation of quinone in the site.
We propose a mechanism in which the water channels allow water molecules to flow into and out of the Qn cavity during catalysis (Fig. 3g). Quantum mechanical calculations indicate that in the absence of substrate (Fig. 3g, state 1), the Qn cavity can be occupied by at least eight water molecules (including wat1), which shield the charged residues—D20b6, K24b6, R207b6, D35sIV and R26PetN (Extended Data Fig. 4)—thereby weakening the interactions between them. This substantially reduces the binding energy of PQ, which, upon entering Qn, pushes the water molecules out of the cavity through the channels in a piston-like manner (Fig. 3g, state 2). The only remaining water molecule is wat1, which allows the quinone ring to adopt an orientation that allows electron and proton transfer to O1 and O4 (Fig. 3g, state 3). When reduction of PQ is complete, PQH2 leaves Qn and exposes charged residues, attracting water molecules into Qn through the channels (Fig. 3g, state 4). The Qn site, filled with water (state 1), is then free to accommodate the next PQ molecule. In this mechanism, synchronized movements of water and PQ/PQH2 facilitate exchange of the substrate/product and thus enable high efficiency of catalytic turnover.
It has been observed that the redox midpoint potential of haem cn shows a strong dependence of −60 mV per pH unit38, which implicates a coupled protonation event. This is not typical for haems in a protein environment. The enhanced exposure of haem cn to water molecules, which can fill the quinone-binding cavity through the water channels, might contribute to the increased pH sensitivity of the redox potential of this haem. In addition, the biphasic titrations of this haem in the presence of NQNO implicate that the occupancy of the Qn site is sensitive to the redox state of the enzyme38. The differences in the density maps of this region in our two samples (1.9 Å and 2.2 Å structure) are in line with these observations. Given that the redox state of haem cn has also been shown to be sensitive to the membrane potential39, the influence of membrane potential on occupancy of the Qn site is to be considered. Haem cn could play a role of the redox sensor to accommodate the catalytic reactions at the Qn site to the dynamically changing redox state of the cells, but such scenarios require further experimental testing.
The proton transfer paths towards O1 and O4 of DPQ described here confirm earlier predictions from analyses of the cytb6f structures with inhibitors bound at Qn (ref. 17). Moreover, our work adds structural details essential for mechanistic considerations. First, wat1 is the primary proton donor for PQ, and it bridges between D35sIV and O1 to facilitate proton transfer. Second, the identified water channels and water-mediated networks of hydrogen bonds implicate a water–PQ exchange mechanism at Qn.
The position of DPQ in our structure differs from the positions described earlier for PQ in cytb6f of plants22 and cyanobacteria24. In those structures, the angles between the PQ ring and the haem cn plane differed from the angles in our structure. Moreover, the PQ ring pointed away from the haem ligand niche, closer to the side of the haem plane and closer to the inter-monomer cavity (Extended Data Fig. 5). Furthermore, in those orientations, PQ was unable to interact with protonable groups in a way that would allow both O1 and O4 to form hydrogen bonds at the same time. As our structure indicates, to achieve such an orientation, the PQ ring must enter the haem cn ligand niche as deeply as the space allows. Incidentally, the PQ ring, in such a position, is relatively close to the protein surface and the cytb6f-bound part of the TSP9 protein for potential interactions with redox partners that are postulated to deliver electrons to Qn from the protein exterior as part of the cyclic electron flow in photosynthesis. The positions of PQ in Qn reported previously might still be relevant to the states during the arrival or departure of PQ to or from the catalytic site.
Interestingly, the TSP9 fragment bound to cytb6f appears to cover the salt bridge formed between the carboxylate group of the C-terminus of cyt b6 and R125sIV. This hinders the accessibility of R125sIV for interaction with a specific kinase involved in state transitions, as part of the regulatory function of cytb6f (refs. 7,40). Furthermore, the carboxyl group of E50TSP9 forms another salt bridge with R125sIV, which additionally stabilizes this conformation (Extended Data Fig. 6). How R125sIV becomes exposed to interact with the kinase remains to be elucidated.
ISP-HD adopts several positions between Qp and haem f
On the other side of the membrane from Qn, the other catalytic site in cytb6f, Qp, oxidizes PQH2 to PQ. For this region, focused three-dimensional (3D) classifications of our cryo-EM datasets identified four distinct classes, representing the most populated states of cytb6f present in the samples (Fig. 4a). These classes correspond to states in which ISP-HD occupies either the b-position (ISP-HD close to cyt b6 and the Qp site), the f-position (ISP-HD close to cyt f, with [2Fe2S] located close to haem f) or an intermediate position. An additional class corresponding to ISP-HD in an intermediate position with PC bound near cyt f was observed. Binding occurs in the same region as previously described21. The presence of these different states in the same dataset confirms the dynamic behaviour of ISP-HD and provides direct structural evidence for its movement, which is postulated to be necessary for catalysis. The b-position seems to reflect the state where electron transfer between 2Fe2S and PQ at the Qp site takes place, and the f-position the state where electron transfer between the [2Fe2S] and haem f takes place (Fig. 4 and Extended Data Fig. 7).
a, Three-dimensional classification of cytb6f particles trapped during catalysis with different ISP-HD and PC positions with percentage results. Top: cryo-EM maps with colour-marked ISP subunit and PC. Bottom: schematic representation of ISP-HD and PC positions in each subgroup of particles. b, ISP-HD position in the cytb6f structure with bound DBMIB (yellow; 2.3 Å; PDB ID: 9ES9) compared with the ISP-HD position from the 1.9 Å structure (green; PDB ID: 9ES7). c, Close-up view of Qp occupied by DBMIB. DBMIB is within hydrogen-bond distance of the Nε atom of the H128 side chain, which also coordinates the 2Fe2S cluster via Nδ.
In spectroscopic studies, the cytb6f inhibitor 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) was found to affect the position of ISP-HD32,41,42; therefore, we used it as a probe to further investigate ISP-HD movement. We determined the structure of DBMIB-inhibited cytb6f at 2.2 Å resolution. In the presence of the inhibitor, only one state of ISP-HD was seen in the dataset. Remarkably, in this structure, ISP-HD was even closer to Qp (Fig. 4b,c). The DBMIB molecule was clearly visible in our reconstruction. A hydrogen bond formed between the carbonyl group of the inhibitor and the Nε atom of H128ISP, which coordinates the 2Fe2S cluster. Y136b6 adopts two conformations (Extended Data Fig. 8a,b), one of which stabilizes DBMIB by forming a second hydrogen bond. In contrast to a previous report43, these data clearly reveal the position of DBMIB in the active site of cytb6f. Consistent with the inhibitory effect of this compound, its influence on the EPR spectrum of the [2Fe2S] cluster and observations based on EPR analysis, DBMIB traps ISP-HD at Qp in the b-position32,41,44. Whether the extremely close position of ISP-HD in complex with DBMIB is a catalytically relevant position remains to be elucidated.
In the DBMIB-bound structure, unlike in other available structures, the first seven amino acids at the N-terminus, preceding the α-helical transmembrane anchor of ISP-HD, become structured (Extended Data Fig. 8c,d). The N-terminus bends towards the protein surface and interacts hydrophobically with cyt f. This change in position of this region might be relevant to cytb6f -mediated redox sensing and signal transduction42,45,46.
In all observed states, the ‘neck’ region that connects ISP-HD to its membrane anchor remains highly unstructured (Extended Data Fig. 9a–c). Only in the cryo-EM map with DBMIB, where the ISP-HD occupies the closest b-position, is the neck region slightly better resolved (Extended Data Fig. 9c). Therefore, the dynamic conformation of the neck region in cytb6f is clearly different from that of cytbc1, which undergoes a sharp transition from a stretched coil-like thread to a proper α-helix when ISP-HD occupies the b-position or the c1-position47 (Extended Data Fig. 9d,e). The lack of an α-helix in the state where ISP-HD is in the f-position (corresponding to the c1-position of cytbc1) implies that the motion of ISP-HD in cytbc1 and cytb6f involves different transitions between conformations.
The position and orientation of the substrate (PQH2) in Qp for the catalytic reaction remain unresolved. In the internal cavities around Qp, both 1.9 Å and 2.2 Å structures show densities remarkably similar to those described in the previous high-resolution structure of cytb6f, which were interpreted as a possible channel for unidirectional PQ traffic through the site21 (Extended Data Fig. 10). Nevertheless, some shortened density fragments might suggest that a partial replacement of natural PQ with the shorter PQ analogue DPQ has taken place. Ligands in the channel remain highly mobile, which makes unambiguous interpretation of their orientation as challenging as in previous analyses.
Conclusion
We present structural and spectroscopic data that point towards fundamentally different binding modes for substrates and inhibitors at the quinone reduction site of cytb6f. Furthermore, we propose a quinone–water exchange mechanism that provides the molecular basis for effective catalytic turnover. Our data reveal overall similarities and minor differences in the motion of ISP-HD in cytb6f compared with cytbc1. The structures also clarify the mechanism of cytb6f inhibition by DBMIB at the Qp site. Our findings contribute to a complete molecular description of the dynamic mechanisms of quinone oxidation and reduction reactions in cytb6f. This work helps us understand the phenomena responsible for photosynthesis and respiration, which enable life on our planet.
Methods
Materials
Buffers, salts and DPQ were purchased from Sigma-Aldrich. Undecyl α-d-maltoside (UDM) was from Glycon. The propyl-sepharose resin was prepared by activating Sepharose-CNBr powder (Cytiva) and reacting it with propylamine (Sigma-Aldrich) according to the supplier’s manual. NQNO (CAS 316-66-5) was purchased from Santa Cruz Biotechnology, and DBMIB (CAS 29096-93-3) was purchased from Sigma-Aldrich.
Purification of PC and cytb 6 f
PC was purified according to the protocol described in ref. 21. To isolate and purify cytb6f from spinach leaves, we used an optimized version of our previously published protocol (protocol 2 from ref. 21). The leaves were processed in a slow juicer with the addition of a cold buffer solution containing 50 mM Tris–HCl pH 8, 200 mM NaCl and 5 mM MgCl2 (buffer 1). The resulting suspension was filtered through a sieve and a bag filter before being subjected to centrifugation at 8,000g for 20 min at 4 °C. The pellet containing chloroplasts was then resuspended in buffer 1 and diluted to a chlorophyll concentration of approximately 2.0 mg ml−1. The chlorophyll content was estimated using the protocol described in ref. 48. The chloroplast suspension was cooled to 4 °C and sonicated with a 500 W ultrasonic processor (VCX 500) equipped with a 13 mm tip. The ultrasonic pulse sequence parameters were set as follows: 50% power, a pulse length of 30 s, a pulse interval of 60 s and a total pulse time of 8 min. Following sonication, the suspension was ultracentrifuged at 148,000g for 20 min at 4 °C. The pellet, consisting of thylakoid membranes, was resuspended in buffer 2 (50 mM Tris–HCl, pH 8.0, 50 mM NaCl), homogenized using a glass homogenizer and diluted to a chlorophyll concentration of 3.0 mg ml−1. Solubilization of the thylakoid membranes was achieved by mixing an equal volume of thylakoid suspension and buffer 2 with the addition of 12 mg ml−1 UDM (final UDM-to-chlorophyll ratio was 4:1). The solution was stirred for 15 min and ultracentrifuged twice at 148,000g for 20 min at 4 °C. To avoid excessively high UDM concentrations in subsequent steps, the supernatant was precipitated with PEG using the following procedure. A solution consisting of 60% (w/w) PEG 4000 in buffer 2 was gradually added to the solubilized thylakoid solution until it became cloudy, resulting in a final PEG concentration of approximately 30%. The resulting suspension was then centrifuged for 10 min at 6,000g at 4 °C, and the supernatant was carefully separated from the pellet. The pellet was collected and solubilized in buffer 2 supplemented with 1 mM UDM (buffer 3). Then, solid ammonium sulfate was added to the solution to achieve 37% ammonium sulfate saturation. The solution was stirred for 15 min, centrifuged at 24,000g for 25 min at 4 °C and filtered through a 0.22 μm syringe filter before loading onto a propyl-sepharose column pre-equilibrated with buffer 3 at 37% ammonium sulfate saturation (buffer 4). The column was washed with 5 column volumes of buffer 4, and the greenish-brown band containing cytb6f was eluted with buffer 3 at 20% ammonium sulfate saturation. The eluent was then brought back to 37% ammonium sulfate, centrifuged at 24,000g for 20 min at 4 °C and filtered through a 0.22 μm syringe filter. The sample was subsequently loaded onto a second propyl-sepharose column pre-equilibrated with buffer 4. The column was washed with 1 column volume of buffer 4 and 5 column volumes of buffer 3 at 30% ammonium sulfate saturation. The brownish band containing cytb6f was eluted with buffer 3 at 25% ammonium sulfate saturation. The collected cytb6f was then pooled, desalted and concentrated using an Amicon Ultra 50 kDa filter. The concentration of cytb6f was assessed by measuring ascorbate-reduced minus ferricyanide-oxidized optical spectra at 554 nm, relative to the isosbestic point at 543 nm, using the differential extinction coefficient of haem f of cyt f ε554–543 ≈ 25 mM−1 cm−1. The sample was loaded onto a 10–22% continuous sucrose gradient made by a freeze–thaw method in buffer 3 and ultracentrifuged at 141,000g for 16 h at 4 °C.
Enzymatic activity measurement
The enzymatic activity of cytb6f was measured spectrophotometrically (SHIMADZU UV-2450) by monitoring the reduction of PC at 597 nm using a procedure similar to that described in ref. 21. Briefly, the experiment was carried out in 50 mM HEPES (pH 7), 50 mM NaCl and 1 mM UDM, with 5 μM oxidized PC present. After initializing the measurement, DPQH2:DPQ (1:1 mixture) was added to the reaction mixture to a final concentration of 10 μM each. Then, cytb6f was added (final concentration of 2 nM) to the mixture to start the enzymatic reaction. The activity of cytb6f was determined from the initial slope of the PC reduction curve, using a PC extinction coefficient ε597 = 4.5 mM−1 cm−1. The activity rate for pure cytb6f was estimated to be 110 s−1. Cytb6f inhibited by DBMIB (cytb6f with a 10-fold excess of DBMIB incubated for 30 min before measurement) was not active.
Preparation of cryo-EM samples
Before the preparation of the cryo-EM samples, purified proteins were transferred to HEPES buffer (50 mM HEPES, 50 mM NaCl, 1 mM UDM, pH 7). For the preparation of DPQ supplemented and catalytic samples, purified cytb6f was concentrated to about 170 μM (calculated as the concentration of cyt f). Then, 53 μl cytb6f stock solution was mixed with 95 μl buffer and 1.5 μl stock DPQ solution (60 mM in DMSO). After about 5 min incubation with DPQ, 3 μl of the sample was applied to the grid and vitrified. For the catalytic sample, 29 μl of oxidized PC (2.1 mM) solution was added to the previously prepared mixture. After a short incubation with PC (about 1 min), 3 μl stock DPQH2 solution (24 mM in EtOH/DMSO) was added. Immediately after DPQH2 addition, 3 μl of the sample was applied to the grid and vitrified. The total time between the addition of DPQH2 and sample vitrification was 15 s, measured using a digital stopwatch. It was verified spectroscopically that at the time of vitrification the PC was not fully reduced in the reaction mixture. Full reduction of PC in that mixture was observed several seconds after vitrification. The sample with DBMIB was prepared separately by mixing 12 μl of cytb6f (153 μM, based on cyt f concentration) with 17 μl of buffer and 1 μl of 50 mM DBMIB. After 5 min incubation, 3 μl of the mixture was applied to the grid and vitrified. In all cases, the blotting and vitrification procedures were the same. Shortly before use, transmission electron microscopy grids (Quantifoil R2/1, Cu 200 mesh) were glow-discharged (60 s, 8 mA). Vitrification was performed on the Vitrobot Mark IV (Thermo Fisher Scientific), and the following parameters were used: 100% humidity, 277 K, 2 s blot time, 0 s wait time, blot force −1, 0 s drain time and a total blot of 1. After plunge-freezing in liquid ethane, the grids were stored in liquid nitrogen until use.
Data acquisition and processing
Cryo-EM data for all samples were collected at the National Cryo-EM Centre SOLARIS in Kraków, Poland. Datasets contained 7,784, 4,613 and 3,027 videos (40 frames each) for samples of cytb6f with DPQ, during catalysis and with DBMIB added. Datasets were collected on a Titan Krios G3i microscope (Thermo Fisher Scientific) at an accelerating voltage of 300 kV, a magnification of ×105,000 and a pixel size of 0.86 Å per pixel (Extended Data Fig. 1a) using EPU 2.10.0.1941REL software. A K3 direct electron detector was used for data collection in a BioQuantum Imaging Filter (Gatan) set-up with a 20 eV slit width. The detector was operated in counting mode. Imaged areas were exposed to 40.7 e− Å−2 (sample with DPQ and frozen during catalysis) or 41.34 e− Å−2 (sample with DBMIB) total dose (corresponding to a dose rate of ~16.62 e− per pixel per second and 15.94 e− per pixel per second measured in vacuum, respectively). The defocus range applied was −2.1 µm to −0.9 µm with 0.3 µm steps. All datasets were processed (Extended Data Fig. 1b) using cryoSPARC v4.4.049,50,51,52,53. Micrographs were motion-corrected using Patch Motion Correction, and the contrast transfer function (CTF) was determined using Patch CTF. In preprocessing, particles from a small portion of micrographs (50–1,000 exposures, depending on dataset quality) were extracted using the Blob picker. The obtained sets of particles were two-dimensionally classified and used for the generation of templates and particle classes for the Template picker and TOPAZ54, respectively. Different approaches for further processing were used depending on the particular dataset quality. In the case of cytb6f with DPQ (Extended Data Fig. 1b, left), the particles obtained from Template picker and TOPAZ were 2D classified and then sorted in ab initio reconstruction. The obtained two pools of particles (1,214,119 and 1,179,618 particles) were merged, and then duplicated particles were removed. The resulting set was cleaned using heterogeneous refinement. Particles from the final set (1,320,164) were extracted into a box of 512 pixels and used for final ab initio reconstruction followed by a non-uniform refinement. The obtained 2.04 Å map was used as an input for reference-based motion correction. The motion-corrected particles (1,320,078) were used for non-uniform refinement with iterative CTF refinement to reconstruct a final structure with 1.94 Å resolution (Extended Data Fig. 1c–f, left). For the catalytic sample of cytb6f with DPQ, PC and DPQH2 (Extended Data Fig. 1b, middle), particles extracted with Template picker were two-dimensionally classified twice and then sorted in ab initio reconstruction and heterogeneous refinement. In the case of particles extracted with TOPAZ, two-dimensional classification was omitted, and they were sorted into five classes in ab initio reconstruction and heterogenous refinement. The best particle sets from the Template picker and TOPAZ picking (251,242 and 490,927 particles, respectively) were pooled together and further sorted in ab initio reconstruction followed by heterogeneous refinement. After the removal of duplicated particles, two extractions were performed. Particles extracted into a box of 512 pixels were used for final ab initio reconstruction followed by non-uniform refinement with iterative CTF refinement. Then, the obtained 2.40 Å map was used as input for reference-based motion correction. The motion-corrected particles (482,950) were used for non-uniform refinement with iterative CTF refinement to reconstruct a final structure with 2.24 Å resolution (Extended Data Fig. 1c–f, middle). The particles extracted into a box of 384 pixels were used for 3D classification. For this purpose, after ab initio reconstruction followed by non-uniform refinement (without iterative CTF refinement, 3.02 Å map resolution), the dataset was symmetry expanded. The particles were then subjected to focused 3D classification with a generous mask encompassing the lumen side of cytochrome f, ISP and bound PC. The 3D classification was initially performed with 2, 4 and 6 classes. However, we found that the effective number of 3D classes is 4. After the removal of duplicate particles, maps were generated by reconstruction only to preserve the prior alignment of particles. In the case of the dataset for cytb6f with DBMIB (Extended Data Fig. 1b, right), particles were first extracted by Template picker, 2D classified and sorted in ab initio reconstruction and heterogenous refinement. The 201,233 particles representing the best class were pooled together with particles picked by two separate TOPAZ models. Then, the particles were subjected to ab initio reconstruction and heterogenous refinement, yielding two good classes of particles, which were then processed further. After the removal of duplicates, the final extraction into a box of 384 pixels was performed, and particles were subjected to two rounds of sorting in ab initio reconstruction and heterogenous refinement. The final set of 391,430 particles was used as input in non-uniform refinement with iterative CTF refinement to reconstruct a 2.38 Å map. Then, the particles were local motion-corrected and used in a final non-uniform refinement with iterative CTF refinement to obtain a final map with 2.33 Å resolution (Extended Data Fig. 1c–f, right). Atomic models were built based on 7ZYV structure from Protein Data Bank (PDB) using ChimeraX55 and Coot56, refined using PHENIX57 and validated with MolProbity58.
EPR spectroscopy
The EPR spectra for all cytb6f samples were measured at 10 K using a Bruker Elexsys E580 spectrometer operating at X band, equipped with a SuperHQ resonator and an ESR900 cryostat (Oxford Instruments). The temperature was set using a Lakeshore 336 temperature controller unit (Lake Shore Cryotronics). The cryogenic temperature was achieved using a Stinger cryocooler (Cold Edge Technologies). The parameters of the measurements were set as follows: microwave frequency, 9.39 GHz; microwave power, 6.35 mW; modulation amplitude, 15 G; modulation frequency, 100 kHz; sweep time, 671 s; sweep width, 4,500 G. Samples of 50 μM cytb6f with and without DPQ added (10× excess) as well as with NQNO added (5× excess) were poised at different ambient redox potential values by adding small aliquots of sodium dithionite solution to an anaerobic, argon-flushed solution. Several redox mediators were used to stabilize the ambient redox potential as described in ref. 59.
QM calculations
The cluster model used in the quantum mechanical calculations was constructed based on the cryo-EM structure of the Qn site not occupied by quinone. The model contains haem cn coordinated by a water molecule, 14 protein residues (D20b6, K24b6, Y25b6 (only backbone fragment), V26b6, I206b6, R207b6, G210b6, I211b6, N25sIV, A31sIV, D35sIV, L36sIV, F40sIV and R26PetN) and 7 or 8 water molecules occupying the Qn site, which were added to fit the density. The geometry optimization was performed using the DFT/B3LYP-D3 method60,61 combined with the def2-SVP basis set62 to determine the position and number of water molecules occupying the active site Qn. The quantum mechanical calculations were performed in Gaussian 16 (ref. 63). During optimization, haem cn with a water ligand and all 14 protein residues were constrained according to the cryo-EM structure, and only the water molecules occupying the Qn site were optimized. Initially, based on the cryo-EM density, seven water molecules were inserted into the Qn site. However, geometry optimization indicated that to maintain the continuity of the water cluster structure, another molecule should be added to the model. Finally, the structure with eight water molecules occupying the Qn site was optimized. The results showed that for oxidized and reduced haem cn (containing high-spin iron) cofactors, the water molecules took similar positions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The micrographs and cryo-EM densities have been deposited in the Electron Microscopy Public Image Archive (EMPIAR) and the Electron Microscopy Data Bank (EMDB) with the following accession codes: EMPIAR-12144, EMPIAR-12158 and EMPIAR-12149, and EMD-19938, EMD-19939 and EMD-19940, respectively. The protein models have been deposited in the PDB and are accessible using the following PDB IDs: 9ES7, 9ES8 and 9ES9. All other data generated in this study are available from corresponding authors on reasonable request.
References
Cramer, W. A. & Kallas, T. (eds) Cytochrome Complexes: Evolution, Structures, Energy Transduction, and Signaling Vol. 41 (Springer, 2016).
Allen, J. F. Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 110, 273–276 (2002).
Sarewicz, M. et al. Catalytic reactions and energy conservation in the cytochrome bc1 and b6f complexes of energy-transducing membranes. Chem. Rev. 121, 2020–2108 (2021).
Mitchell, P. The protonmotive Q cycle: a general formulation. FEBS Lett. 59, 137–139 (1975).
Hasan, S. S., Yamashita, E. & Cramer, W. A. Transmembrane signaling and assembly of the cytochrome b6f-lipidic charge transfer complex. Biochim. Biophys. Acta 1827, 1295–1308 (2013).
Tikhonov, A. N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 81, 163–183 (2014).
Dumas, L. et al. A stromal region of cytochrome b6f subunit IV is involved in the activation of the Stt7 kinase in Chlamydomonas. Proc. Natl Acad. Sci. USA 114, 12063–12068 (2017).
Nawrocki, W. J. et al. The mechanism of cyclic electron flow. Biochim. Biophys. Acta 1860, 433–438 (2019).
Crofts, A. R., Meinhardt, S. W., Jones, K. R. & Snozzi, M. The role of the quinone pool in the cyclic electron-transfer chain of Rhodopseudomonas sphaeroides A modified Q-cycle mechanism. Biochim. Biophys. Acta 723, 202–218 (1983).
Cape, J. L., Bowman, M. K. & Kramer, D. M. Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends Plant Sci. 11, 46–55 (2006).
Malone, L. A., Proctor, M. S., Hitchcock, A., Hunter, C. N. & Johnson, M. P. Cytochrome b6f—orchestrator of photosynthetic electron transfer. Biochim. Biophys. Acta 1862, 148380 (2021).
Berry, E. A. et al. X-ray structure of Rhodobacter capsulatus cytochrome bc1: comparison with its mitochondrial and chloroplast counterparts. Photosynth. Res. 81, 251–275 (2004).
Gao, X. et al. Structural basis for the quinone reduction in the bc1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry 42, 9067–9080 (2003).
Iwata, S. et al. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281, 64–71 (1998).
Stroebel, D., Choquet, Y., Popot, J.-L. & Picot, D. An atypical haem in the cytochrome b6f complex. Nature 426, 413–418 (2003).
Kurisu, G., Zhang, H., Smith, J. L. & Cramer, W. A. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302, 1009–1014 (2003).
Hasan, S. S., Yamashita, E., Baniulis, D. & Cramer, W. A. Quinone-dependent proton transfer pathways in the photosynthetic cytochrome b6f complex. Proc. Natl Acad. Sci. USA 110, 4297–4302 (2013).
Hasan, S. S., Proctor, E. A., Yamashita, E., Dokholyan, N. V. & Cramer, W. A. Traffic within the cytochrome b6f lipoprotein complex: gating of the quinone portal. Biophys. J. 107, 1620–1628 (2014).
Hasan, S. S. & Cramer, W. A. Internal lipid architecture of the hetero-oligomeric cytochrome b6f complex. Structure 22, 1008–1015 (2014).
Baniulis, D. et al. Structure-function, stability, and chemical modification of the cyanobacterial cytochrome b6f complex from Nostoc sp. PCC 7120. J. Biol. Chem. 284, 9861–9869 (2009).
Sarewicz, M. et al. High-resolution cryo-EM structures of plant cytochrome b6f at work. Sci. Adv. 9, eadd9688 (2023).
Malone, L. A. et al. Cryo-EM structure of the spinach cytochrome b6f complex at 3.6 Å resolution. Nature 575, 535–539 (2019).
Yamashita, E., Zhang, H. & Cramer, W. A. Structure of the cytochrome b6f complex: quinone analogue inhibitors as ligands of heme cn. J. Mol. Biol. 370, 39–52 (2007).
Proctor, M. S. et al. Cryo-EM structures of the Synechocystis sp. PCC 6803 cytochrome b6f complex with and without the regulatory PetP subunit. Biochem. J. 479, 1487–1503 (2022).
Roberts, A. G., Bowman, M. K. & Kramer, D. M. The inhibitor DBMIB provides insight into the functional architecture of the Qo site in the cytochrome b6f complex. Biochemistry 43, 7707–7716 (2004).
Darrouzet, E., Valkova-Valchanova, M., Moser, C. C., Dutton, P. L. & Daldal, F. Uncovering the [2Fe2S] domain movement in cytochrome bc1 and its implications for energy conversion. Proc. Natl Acad. Sci. USA 97, 4567–4572 (2000).
Tian, H., White, S., Yu, L. & Yu, C.-A. Evidence for the head domain movement of the Rieske iron–sulfur protein in electron transfer reaction of the cytochrome bc1 complex. J. Biol. Chem. 274, 7146–7152 (1999).
Sarewicz, M., Dutka, M., Froncisz, W. & Osyczka, A. Magnetic interactions sense changes in distance between heme bL and the iron−sulfur cluster in cytochrome bc1. Biochemistry 48, 5708–5720 (2009).
Cooley, J. W., Roberts, A. G., Bowman, M. K., Kramer, D. M. & Daldal, F. The raised midpoint potential of the [2Fe2S] cluster of cytochrome bc1 is mediated by both the Qo site occupants and the head domain position of the Fe−S protein subunit. Biochemistry 43, 2217–2227 (2004).
Zhang, Z. et al. Electron transfer by domain movement in cytochrome bc1. Nature 392, 677–684 (1998).
Bowman, M. K., Berry, E. A., Roberts, A. G. & Kramer, D. M. Orientation of the g-tensor axes of the Rieske subunit in the cytochrome bc1 complex. Biochemistry 43, 430–436 (2004).
Roberts, A. G., Bowman, M. K. & Kramer, D. M. Certain metal ions are inhibitors of cytochrome b6f complex ‘Rieske’ iron−sulfur protein domain movements. Biochemistry 41, 4070–4079 (2002).
Rao, B. K. S., Tyryshkin, A. M., Roberts, A. G., Bowman, M. K. & Kramer, D. M. Inhibitory copper binding site on the spinach cytochrome b6f complex: implications for Qo site catalysis. Biochemistry 39, 3285–3296 (2000).
de Lacroix de Lavalette-Boehm, A., Barucq, L., Alric, J., Rappaport, F. & Zito, F. Is the redox state of the ci heme of the cytochrome b6f complex dependent on the occupation and structure of the Qi site and vice versa? J. Biol. Chem. 284, 20822–20829 (2009).
Zatsman, A. I., Zhang, H., Gunderson, W. A., Cramer, W. A. & Hendrich, M. P. Heme–heme interactions in the cytochrome b6f complex: EPR spectroscopy and correlation with structure. J. Am. Chem. Soc. 128, 14246–14247 (2006).
Baymann, F., Giusti, F., Picot, D. & Nitschke, W. The ci/bH moiety in the b6f complex studied by EPR: a pair of strongly interacting hemes. Proc. Natl Acad. Sci. USA 104, 519–524 (2007).
Szwalec, M., Bujnowicz, Ł., Sarewicz, M. & Osyczka, A. Unexpected heme redox potential values implicate an uphill step in cytochrome b6f. J. Phys. Chem. B 126, 9771–9780 (2022).
Alric, J., Pierre, Y., Picot, D., Lavergne, J. & Rappaport, F. Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc. Natl Acad. Sci. USA 102, 15860–15865 (2005).
Lavergne, J. Membrane potential-dependent reduction of cytochrome b-6 in an algal mutant lacking photosystem I centers. Biochim. Biophys. Acta 725, 25–33 (1983).
Riché, A. et al. The stromal side of cytochrome b6f complex regulates state transitions. The Plant Cell koae190 (2024); https://doi.org/10.1093/plcell/koae190
Schoepp, B., Brugna, M., Riedel, A., Nitschke, W. & Kramer, D. M. The Qo-site inhibitor DBMIB favours the proximal position of the chloroplast Rieske protein and induces a pK-shift of the redox-linked proton. FEBS Lett. 450, 245–250 (1999).
Vener, A. V., van Kan, P. J. M., Gal, A., Andersson, B. & Ohad, I. Activation/deactivation cycle of redox-controlled thylakoid protein phosphorylation: role of PLASTOQUINOL bound to the reduced cytochrome bf complex. J. Biol. Chem. 270, 25225–25232 (1995).
Yan, J., Kurisu, G. & Cramer, W. A. Intraprotein transfer of the quinone analogue inhibitor 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone in the cytochrome b6f complex. Proc. Natl Acad. Sci. USA 103, 69–74 (2006).
Sarewicz, M. et al. Metastable radical state, nonreactive with oxygen, is inherent to catalysis by respiratory and photosynthetic cytochromes bc1/b6f. Proc. Natl Acad. Sci. USA 114, 1323–1328 (2017).
Rinalducci, S., Larsen, M. R., Mohammed, S. & Zolla, L. Novel protein phosphorylation site identification in spinach stroma membranes by titanium dioxide microcolumns and tandem mass spectrometry. J. Proteome Res. 5, 973–982 (2006).
Zito, F. et al. The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J. 18, 2961–2969 (1999).
Cooley, J. W. Protein conformational changes involved in the cytochrome bc1 complex catalytic cycle. Biochim. Biophys. Acta 1827, 1340–1345 (2013).
Porra, R. J., Thompson, W. A. & Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394 (1989).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Rubinstein, J. L. & Brubaker, M. A. Alignment of cryo-EM movies of individual particles by optimization of image translations. J. Struct. Biol. 192, 188–195 (2015).
Stagg, S. M., Noble, A. J., Spilman, M. & Chapman, M. S. ResLog plots as an empirical metric of the quality of cryo-EM reconstructions. J. Struct. Biol. 185, 418–426 (2014).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Bepler, T., Kelley, K., Noble, A. J. & Berger, B. Topaz-Denoise: general deep denoising models for cryoEM and cryoET. Nat. Commun. 11, 5208 (2020).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Sarewicz, M., Pintscher, S., Bujnowicz, Ł., Wolska, M. & Osyczka, A.The high-spin heme bL mutant exposes dominant reaction leading to the formation of the semiquinone spin-coupled to the [2Fe-2S]+ cluster at the Qo site of Rhodobacter capsulatus cytochrome bc1. Front. Chem. 9, 658877 (2021).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, 2016).
Acknowledgements
We thank all members of the Osyczka and Glatt laboratories for discussion and suggestions. This work was supported by the Team grant (POIR.04.04.00-00-5B54/17-00 to A.O.) and the Team Tech core facility grant (TEAM TECH CORE FACILITY/2017-4/6 to S.G.) from the Foundation for Polish Science, and the OPUS-24 grant (2022/47/B/NZ1/03308 to A.O.) from NCN. The work is supported under the Polish Ministry of Education and Science project: ‘Support for research and development with the use of research infrastructure of the National Synchrotron Radiation Centre SOLARIS’ under contract number 1/SOL/2021/2. We also acknowledge the Polish high-performance computing infrastructure PLGrid (HPC Center: ACK Cyfronet AGH) for providing computer facilities and support within computational grant numbers PLG/2022/015736, PLG/2022/015891 and PLG/2023/016614. The open access of this article was funded by the Priority Research Area BioS under the program ‘Initiative of Excellence - Research University’ at the Jagiellonian University in Kraków. We also acknowledge infrastructural support from this programme.
Author information
Authors and Affiliations
Contributions
The study was designed and directed by S.G. and A.O. R.P. and B.M. isolated and purified cytb6f complexes. S.P. isolated and purified PC. S.P., R.P. and B.M. prepared cryo-EM samples. G.W. and S.P. performed initial grid screening and grid optimization. Data collection was done by M.J., P.I., G.W. and M.R. Data processing was done by S.P., M.J. and Ł.K. Map interpretation, model building and refinement were done by S.P. and R.P. with the help of M.J., Ł.K. and S.G. M.S. performed EPR experiments. A.W.-A. performed QM calculations and analysis. Figures were prepared by S.P., R.P. and B.M. with support from S.G. and A.O. The paper was written by S.P., R.P., S.G. and A.O., with all authors discussing the results and refining and approving the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Jean Alric and Michael Hippler for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM data processing workflow.
Cryo-EM datasets processing for cytb6f with DPQ (1.9 Å map; EMD-19938), cytb6f frozen under turnover conditions (2.2 Å map; EMD-19939) and cytb6f with DBMIB (2.3 Å map; EMD-19940); left, middle and right, respectively. a, representative cryo-EM fields; b, cryoSPARC processing pipelines; c, Fourier shell correlation curves, dark red lines in the plots indicate FSC = 0.143; d, local-filtered maps colored by resolution range; e, particle angular distribution heatmaps calculated in cryoSPARC; f, selected 2D class averages for the particles contributing to the respective maps.
Extended Data Fig. 2 Additional views on quinone in Qn site of cytb6f.
Additional close-up views on quinone in Qn of cytb6f taken from 2.2 Å structure, PDB ID: 9ES8. a, ring of hydrophobic sidechains around wat1 and D35sIV; b, the hydrophilic environment around one of the haem cn propionates groups (‘distal propionate’).
Extended Data Fig. 4 Water molecules in Qn site predicted from QM simulation.
Geometry of QM model with optimized positions of eight water molecules at Qn.
Extended Data Fig. 7 The movement of iron-sulphur protein head domain.
Distances between 2Fe2S cluster and haem f and haem bp for different positions of ISP-HD. a, for the subgroup with ISP-HD at b-position; b, for the subgroup with ISP-HD at intermediate position; c, for the subgroup with ISP-HD at f-position; d, for the subgroup with ISP-HD at intermediate position with PC bound (note that position of ISP-HD is as in subgroup shown in b and the only difference is the presence of PC).
Extended Data Fig. 8 Structural features in cytb6f with DBMIB bound.
Alternative conformations of Y136b6 in cytb6f structure with DBMIB (PDB ID: 9ES9). The Y136b6 side-chain is placed away from or close to the DBMIB molecule (a and b, respectively). Close-up view on the membranous n-side region around the N-terminus of the ISP subunit in 1.9 Å structure (PDB ID: 9ES7) (c) and the structure with DBMIB (PDB ID: 9ES9) (d).
Extended Data Fig. 9 Comparison of ISP neck region structure between cytb6f and cytbc1.
a, and b, cryo-EM maps obtained from 3D classification of cytb6f particles frozen during catalysis (ISP head domain was in f- or b-position, respectively). The low intensity of the map of the ISP neck (sequence: GGAGTG) indicates high flexibility of the peptide backbone in this region. c, When DBMIB is bound to cytb6f (PDB ID: 9ES9), the ISP locks in b-position and the ISP neck becomes more rigid and this region is better resolved. The ISP neck in cytbc1 behaves differently: when ISP is in c-position the ISP neck forms α-helix14 (d), when ISP is in the b-position, the helix is stretched13 (e).
Extended Data Fig. 10 Quinone channel in cytochrome b6f.
Cryo-EM map densities in the PQ channel area at the Qp side in two maps reported in this paper (top) compared to our previous report (bottom).
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pintscher, S., Pietras, R., Mielecki, B. et al. Molecular basis of plastoquinone reduction in plant cytochrome b6f. Nat. Plants 10, 1814–1825 (2024). https://doi.org/10.1038/s41477-024-01804-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41477-024-01804-x
This article is cited by
-
Structure, regulation and assembly of the photosynthetic electron transport chain
Nature Reviews Molecular Cell Biology (2025)