Abstract
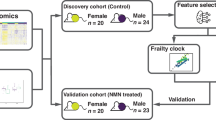
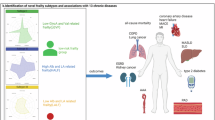
Frailty is a common geriatric syndrome associated with increased mortality, yet its underlying biological mechanisms and potential value for early risk stratification remain inadequately understood. In this large prospective cohort of more than 260,000 UK Biobank participants with plasma metabolomic profiling, we identified and validated metabolomic signatures of physical frailty and a 49-item frailty index using 50-times repeated 10-fold cross-validated elastic-net regression. The signatures demonstrated strong internal stability and geographic reproducibility and reflected coordinated alterations across lipid, amino acid, energy, and inflammatory pathways. Higher signature levels were significantly associated with increased risks of all-cause and cause-specific mortality, including cancer, cardiovascular, respiratory, and digestive deaths. Individuals in the highest-risk tertile had more than 2.5-fold higher risks of cardiovascular, respiratory, and digestive mortality. At age 60, individuals above the median signature level were estimated to have 4.1 fewer years of life expectancy. Mediation analyses indicated that the metabolomic signatures statistically explained up to 35% of the observed frailty–mortality association. Associations were stronger among younger individuals and differed by sex and BMI. These findings suggest that frailty-related plasma metabolomic signatures capture systemic metabolic correlates of biological aging and may support early mortality risk prediction and personalized prevention strategies in aging populations.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the UK Biobank (https://www.ukbiobank.ac.uk/), but restrictions apply to their availability. The data were used under licence for the current study and are therefore not publicly available. Access to the UK Biobank resource requires an approved application; researchers may apply for data access through the UK Biobank Access Management System.
References
Kim, D. H. & Rockwood, K. Frailty in older adults. N. Engl. J. Med 391, 538–548 (2024).
Hoogendijk, E. O. et al. Frailty: Implications for clinical practice and public health. Lancet 394, 1365–1375 (2019).
Dent, E. et al. Management of frailty: Opportunities, challenges, and future directions. Lancet 394, 1376–1386 (2019).
Hanlon, P. et al. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493,737 UK Biobank participants. Lancet Public Health 3, e323–e332 (2018).
Wen, L. et al. Association of frailty and pre-frailty with all-cause and cardiovascular mortality in diabetes: Three prospective cohorts and a meta-analysis. Ageing Res Rev. 106, 102696 (2025).
Peng, Y., Zhong, G.-C., Zhou, X., Guan, L. & Zhou, L. Frailty and risks of all-cause and cause-specific death in community-dwelling adults: A systematic review and meta-analysis. BMC Geriatr. 22, 725 (2022).
Fan, J. et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: A prospective cohort study. Lancet Public Health 5, e650–e660 (2020).
Kojima, G., Iliffe, S. & Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 47, 193–200 (2018).
Jiang, M. et al. Frailty index as a predictor of all-cause and cause-specific mortality in a Swedish population-based cohort. Aging 9, 2629–2646 (2017).
Yang, Y., Chen, L. & Filippidis, F. T. Accelerometer-measured physical activity, frailty, and all-cause mortality and life expectancy among middle-aged and older adults: A UK biobank longitudinal study. BMC Med. 23, 125 (2025).
Ida, S., Kaneko, R., Imataka, K. & Murata, K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: A systematic review and meta-analysis. Cardiovasc Diabetol. 18, 81 (2019).
Fried, L. P. et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med Sci. 56, M146–M156 (2001).
Fried, L. P. et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 1, 36–46 (2021).
Williams, D. M., Jylhävä, J., Pedersen, N. L. & Hägg, S. A frailty index for UK biobank participants. J. Gerontol. A Biol. Sci. Med Sci. 74, 582–587 (2019).
Ofori-Asenso, R. et al. Global incidence of frailty and prefrailty among community-dwelling older adults: A systematic review and meta-analysis. JAMA Netw. Open 2, e198398 (2019).
Walsh, B. et al. Frailty transitions and prevalence in an ageing population: Longitudinal analysis of primary care data from an open cohort of adults aged 50 and over in England, 2006-2017. Age Ageing 52, afad058 (2023).
Gale, C. R., Cooper, C. & Sayer, A. A. Prevalence of frailty and disability: Findings from the English longitudinal study of ageing. Age Ageing 44, 162–165 (2015).
Kameda, M., Teruya, T., Yanagida, M. & Kondoh, H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc. Natl. Acad. Sci. USA 117, 9483–9489 (2020).
Dzięgielewska-Gęsiak, S. & Muc-Wierzgoń, M. Inflammation and oxidative stress in frailty and metabolic syndromes-two sides of the same coin. Metabolites 13, 475 (2023).
Saedi, A. A., Feehan, J., Phu, S. & Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin. Inter Aging 14, 389–398 (2019).
Shrauner, W. et al. Frailty and cardiovascular mortality in more than 3 million US veterans. Eur. Heart J. 43, 818–826 (2022).
Zhang, X.-R. et al. Improved prediction and risk stratification of major adverse cardiovascular events using an explainable machine learning approach combining plasma biomarkers and traditional risk factors. Cardiovasc Diabetol. 24, 153 (2025).
Wang, S. et al. Mitochondria-derived methylmalonic acid, a surrogate biomarker of mitochondrial dysfunction and oxidative stress, predicts all-cause and cardiovascular mortality in the general population. Redox Biol. 37, 101741 (2020).
Musso, G., Cassader, M., Paschetta, E. & Gambino, R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 155, 282–302.e8 (2018).
Llauradó, G. et al. Measurement of serum N-glycans in the assessment of early vascular aging (arterial stiffness) in adults with type 1 diabetes. Diab Care 45, 2430–2438 (2022).
Chen, Y.-F. et al. n-3 polyunsaturated fatty acids in phospholipid or triacylglycerol form attenuate nonalcoholic fatty liver disease via mediating cannabinoid receptor 1/adiponectin/ceramide pathway. J. Nutr. Biochem 123, 109484 (2024).
Buergel, T. et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med. 28, 2309–2320 (2022).
Zhang, P.-D. et al. Associations of genetic risk and smoking with incident COPD. Eur. Respir. J. 59, 2101320 (2022).
Mitnitski, A. B., Mogilner, A. J. & Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World JOURNAL 1, 323–336 (2001).
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. & Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008).
Julkunen, H. et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK biobank. Nat. Commun. 14, 604 (2023).
Li, Z.-H. et al. Associations of regular glucosamine use with all-cause and cause-specific mortality: A large prospective cohort study. Ann. Rheum. Dis. 79, 829–836 (2020).
Zhu, K. et al. Proteomic signatures of healthy dietary patterns are associated with lower risks of major chronic diseases and mortality. Nat. Food 6, 47–57 (2025).
Buuren, van, S., Groothuis-Oudshoorn & mice:, K. Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 45, 1–67 (2011).
Trevor Hastie, Robert Tibshirani, & Jerome Friedman. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. (Springer New York Inc, New York, 2009).
Szklo, M. & Nieto, F. J. Epidemiology: Beyond the Basics. (Jones & Bartlett Learning, Burlington, Mass, 2014).
Celentano, D. D. & Szklo, M. Gordis Epidemiology. (Elsevier, lnc, Philadelphia, PA 19103-2899 USA, 2018).
Hennekens, Charles H. & Buring, Julie E. Epidemiology in Medicine. (Lippincott Williams & Wilkins, Philadelphia, PA 19106 USA, 1987).
Chudasama, Y. V. et al. Physical activity, multimorbidity, and life expectancy: A UK biobank longitudinal study. BMC Med. 17, 108 (2019).
Dehbi, H.-M., Royston, P. & Hackshaw, A. Life expectancy difference and life expectancy ratio: Two measures of treatment effects in randomised trials with non-proportional hazards. BMJ 357, j2250 (2017).
Kulesa, A., Krzywinski, M., Blainey, P. & Altman, N. Sampling distributions and the bootstrap. Nat. Methods 12, 477–478 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Acknowledgements
This study was conducted using data from the UK Biobank resource under application number 98679. We are grateful to all participants and professionals contributing to the UK Biobank. This work was supported by the National Natural Science Foundation of China to X.Z. (82304211), C.M. (82425052), C.D. (82271298), and X.F. (82201427), and by the Foundation of the National Health Commission Capacity Building and Continuing Education Center to C.D. (GWJJ2022100102). The funders had no role in the study design or conduct; data collection, management, analysis, or interpretation; manuscript preparation, review or approval; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
X.Z. and X.F. conceived and designed the study and contributed equally to this work. C.M. and C.D. supervised the study. X.Z., X.F., and Q.H. acquired, analyzed, and interpreted the data. X.Z., P.Z., and Z.L. provided statistical expertise. C.M., C.D., X.Z., X.F., W.L., and Q.H. contributed to the discussion and interpretation of the results. X.Z. and R.L. drafted the manuscript. All authors critically revised the manuscript for important intellectual content, approved the final version, and agreed to be accountable for all aspects of the work. C.M., C.D., X.Z., and X.F. secured funding. X.F. and Z.L. provided technical, material, or administrative support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Feng, X., Liu, W. et al. Frailty-related plasma metabolomic signatures predict long-term mortality risk and implicate systemic aging pathways: evidence from a prospective cohort study. npj Aging (2026). https://doi.org/10.1038/s41514-025-00327-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-025-00327-9