Abstract
In exploring a growing demand for innovative approaches to tackle emerging and life threatening fungal diseases, we identified long-chain 4-aminoquinoline (4-AQ) derivatives as a new class of anti-virulence agents. For the first time, we demonstrated that 4-AQs effectively prevent filamentation of Candida albicans, a key virulence trait, under multiple triggering conditions. Selected 4-AQ derivatives inhibited filament formation in a zebrafish model of disseminated candidiasis at 1.56 µM, with no toxicity up to 50 µM. Combining nystatin with 4-AQs resulted in a 100% survival rate of infected embryos and complete eradication of C. albicans, compared to 65–75% survival with nystatin alone. The most potent 4-AQ derivatives also showed significant activity against C. albicans biofilms, with derivative 11 suppressing mixed C. albicans-Pseudomonas aeruginosa biofilms. This dual capability highlights the potential of 4-AQs as novel anti-virulence agents to enhance conventional antifungal therapies, marking a significant advance in treating complex fungal infections.
Similar content being viewed by others
Introduction
In the evolving landscape of infectious diseases, the prevalence and complexity of fungal infections has changed significantly in the past decades. Once considered a rare disease, these infections have become an everyday problem in modern societies, afflicting afflict over a billion people annually, with 1.6 million deaths1,2. Among the 600 fungal species known to infect humans, Candida species, particularly Candida albicans, account for more than 85% of fungemia cases and pose a serious threat to human health worldwide2.
C. albicans, typically a commensal fungus in the microbiota of the human skin, gastrointestinal tract and urogenital tract, has emerged as a dreaded pathogen responsible for a spectrum of diseases, known as candidiasis2. These infections are particularly prevalent among immunocompromised individuals (HIV/AIDS, cancer, diabetes) and those undergoing aggressive immunosuppressive therapy (chemotherapy, radiation, corticosteroids, organ transplantation). Recent epidemiological surveys documented several million cases of mucosal candidiasis (superficial, oropharyngeal, pulmonary and vaginal) and 700,000 cases of life-threatening invasive (systemic) candidiasis1, the incidence of which increased significantly during the COVID-19 pandemic3. Recognizing the critical threat of fungal infections, the WHO published the first global list of priority fungal pathogens in 2022, in which C. albicans was ranked with the highest public health importance4.
Despite the high prevalence of fungal infections, the treatments for candidiasis are limited to four classes of approved drugs—polyenes, azoles, echinocandins and pyrimidines, the most recent class having been discovered over three decades ago5,6. Existing therapies are often only partially effective and fail to completely eliminate fungal infections. There are several reasons for this, including subtherapeutic drug concentration at the site of infection and suboptimal pharmacokinetics in a physiological environment (e.g., in blood serum), a small number of fungal-specific targets and a limited spectrum of activity, coupled with limited routes of administration (echinocandins) and severe side effects ranging from hepatotoxicity (azoles, echinocandins, flucytosine and amphotericin B formulations) and nephrotoxicity (amphotericin B) to systemic toxicity (polyenes)7. To make matters worse, increasing antifungal resistance, enforced by the fungicidal and fungistatic nature of existing drugs, further compromises their efficacy and poses a major clinical challenge8. These factors emphasize the urgent need for new antifungal agents and innovative therapeutic approaches.
A promising therapeutic strategy that has attracted a huge attention focuses on targeting fungal virulence rather than its viability, aiming to disarm the pathogen, prevent infected tissue damages and enhance drug efficacy 9,10. A key aspect of the virulence of C. albicans is its ability to transition from yeast to a filamentous form, and plays a crucial role in establishing infections. This morphogenetic switch, characterized by forming elongated hyphae, increases the fungus’ s virulence enabling disruption of epithelial barriers, host tissues invasion and immune escape, leading to life-threatening systemic infections11. Hyphae are also crucial for the formation of biofilm, which are difficult-to-treat microbial structures with a high resistance to clinical drugs. This morphological plasticity of C. albicans significantly complicates antifungal treatment and contributes to the severity and persistence of candidiasis. On the other hand, animal studies noticed that the strains locked in a yeast form are avirulent or exhibit reduced virulence. Accordingly, an anti-virulence agent is expected to increase the efficacy of antifungal treatments by increasing the pathogen sensitivity to administered drugs, reducing required drug doses while reducing/preventing the development of resistance and thus, ensuring complete eradication of fungal infection12.
In chronic infections, Candida often coexists in mixed communities with various bacteria such as Pseudomonas aeruginosa, an opportunistic Gram-negative bacterium also classified as a high-priority pathogen by the WHO13. These two superbugs are the most commonly isolated pathogens of mixed (polymicrobial) infections (cystic fibrosis, burns, diabetic ulcers, otitis media, medical devices, ICU, immunocompromised patients)14,15,16,17, in which they produce intra-species signaling molecules that also serve for inter-kingdom crosstalk to regulate virulence and pathogenicity. Pseudomonas quinolone signaling (PQS) and its immediate precursor 2-heptyl-4-hydroxyquinoline (HHQ) are quorum-sensing signaling molecules that control the production of secondary metabolites in P. aeruginosa and the synthesis of several virulence factors such as elastase, pyocyanin, motility and biofilm18. Accordingly, we have recently developed a series of structural HHQ analogs, namely long-chain 4-aminoquinoline derivatives (4-AQ) that interfere with the PQS system and inhibit the multiple virulence traits of P. aeruginosa19. Considering the great demand for new antifungal agents and treatment strategies as well as the role of HHQ in the inhibition of C. albicans biofilm20, in this study we systematically investigated the potential of the 4-AQ derivatives to suppress C. albicans virulence in vitro and in vivo, to enable effective eradication of the fungal infection in combination with the antifungal drugs nystatin and finally to be used in the treatment of mixed P. aeruginosa-C. albicans biofilm infections.
Results
Identification of the 4-AQ molecules with anti-virulence activity against C. albicans
Quinolines have attracted increasing attention in the search for new therapeutic agents with a broad spectrum of pharmacological activities21. Although the quinoline ring represents an important scaffold for the development of new antifungal molecules, their potential to suppress fungal virulence is still largely unexplored, especially the effect on the development of C. albicans hyphae, the major virulence trait of this pathogenic fungus.
In previous studies, we reported the synthesis and chemical characterization of long-chain 4-AQ derivatives that inhibit multiple virulence factors in P. aeruginosa, such as pyocyanin synthesis, elastase production, motility, and biofilm formation, by interfering with bacterial PQS pathway19. To unveil whether these 4-AQ molecules also possess anti-virulence activity against C. albicans, in this study we screened sixteen derivatives (Supplementary table 2, Fig. 1a) for their antifungal activity on the three reference strains, C. albicans SC5314, C. albicans ATCC10231 and C. glabrata ATCC2001, aiming to distinguish the molecules with antifungal activity from those with anti-virulence activity and no effect on pathogen growth. The activity of the 4-AQs was compared with that of amphotericin B, nystatin and itraconazole (clinical drugs used for mucosal and/or systemic candidiasis treatment) and HHQ (a known inhibitor of the C. albicans biofilm)20,22. In parallel, we systematically investigated the toxicity of all 4-AQ compounds, HHQ and antifungal drugs in vivo using the zebrafish model (Danio rerio), by assessing their effect on the embryos survival, the appearance of teratogenic malformations and signs of cardiotoxicity and hepatotoxicity (Supplementary Table 1). The zebrafish has proven to be a universal biotechnological platform for the assessment of bioactivity and toxicity of chemical diversity due to its high molecular-genetic, physiological and immunological similarity to humans, and the high correlation in pharmacological response to xenobiotics23,24. Employing this model system early in preclinical research simplifies the path to clinical trials while reducing the risk of failures in later testing phases25.
a The chemical structures of HHQ (2-heptyl-4-hydroxyquinoline) and selected 4-AQ molecules differing in a group bound to the side chain (in blue) and their anti-virulence activities on P. aeruginosa PAO1. b Heatmap illustrating the hierarchical clustering of 4-AQs, HHQ and clinical antifungals, classified using Euclidean distance and Ward’s method into three distinct groups based on their similarity in inhibitory activity against three Candida strains (average of two independent experiments) and toxicity profiles in zebrafish embryos (n = 20 per concentration, tested in triplicate). c The normally developing zebrafish embryos without the signs of toxicity after 5-day exposure to 4-AQ derivatives 11 (Group II), and 6 and 24 (Group III) are shown. In contrast, the embryos treated with 2 µM nystatin (Group I) and 5 µM HHQ were severely damaged and suffered from liver necrosis (dashed line outlining the dark liver), impaired yolk uptake (asterisk), life-threatening pericardial edema (arrow), jaw deformation (arrowhead) and head deformation (bracket). d Heatmap showing the four groups of molecules within the 4-AQs series that differ in their filamentation inhibitory activity (the number of media in which filament formation was completely blocked). After fungal cells were exposed to 10 µM of each molecule for 4-6 days and colony morphology was examined microscopically, each molecule was scored binary for its effect on filament development (presence or absence of filaments) and hierarchically clustered using Ward’s clustering method with Euclidean distance. e The representative images showing the morphology of the fungal colonies after the 3-day treatment with a dose of 10 µM of the selected 4-AQ derivatives (6, 11 and 24). Robust filaments and/or wrinkled colonies were developed in control (0.02% DMSO) treatment, while active molecules inhibited the formation of filaments and/or caused smooth colonies. Hierarchical clustering was performed using the Euclidian distance and Ward’s method.
The data obtained showed that majority of the 4-AQ derivatives, similar to HHQ, had no effect on Candida growth (MIC ≥ 100 µM), while the active derivatives (12, 13, 16, 17, 19 and 25) had MIC values between 25 and 50 µM (Fig. 1a, Supplementary table 2), indicating weak to moderate antifungal activity. To accurately determine the similarity between the tested molecules and the diversity within the 4-AQ series based on their bioactivity (antifungal effect and in vivo toxicity), all tested molecules were hierarchically classified using Ward’s clustering method, grouping molecules with similar bioactivity profiles together. This analysis showed three distinct groups among the molecules tested (Fig. 1b) - Group I comprised clinical drugs that were active against all Candida strains with very low MIC values (MIC = 0.19–1 µM), Group II included 4-AQ molecules with weak-to-moderate antifungal activity (MIC = 25–50 µM), while the compounds that had no effect on the growth of the tested fungal strains (MIC ≥ 100 µM) formed Group III.
It is important to note that the majority of 4-AQ derivatives, including those with antifungal activity (12, 17, and 25; Group II) and those without antifungal activity (10, 11, 22, 23, 24, and 28; Group III), did not elicit any toxic response in vivo at doses up to 50 µM (Fig. 1b, c), indicating a good safety profile of these 4-AQ molecules. Moreover, no side effects were observed even at 100 µM (data not shown). In contrast, antifungal drugs at effective (MIC) doses and HHQ at low doses (≥3.12 µM) caused significant side effects, particularly affecting liver, cardiovascular functions and skeletal development (Fig. 1c), reflecting safety issues observed in clinical practice or hindering the possibility for clinical application. Overall, these data demonstrated that the 4-AQ derivatives, representing structural analogs of HHQ, have a desirable safety profile that warrants further investigation of their potential use as anti-virulence agents.
Inhibition of filamentation in C. albicans
The yeast-to-hyphae morphogenetic switch is a critical virulence factor that enables C. albicans to invade host tissues and establish infections in diverse human body niches, such as the oral cavity, skin, lungs, vagina, and gastrointestinal tract26. Although quinoline-based scaffolds have been extensively explored in antifungal drug development, no studies to date have addressed their potential impact on C. albicans virulence traits or their modulation of the underlying biochemical pathways. Accordingly, we evaluated the anti-filamentation potential of 4-AQ derivatives under several triggering stimuli to which the fungus may be exposed during colonization of the different niches in the human body. The anti-virulence activity was tested on C. albicans SC5314, as the biochemical pathways governing filamentation in this fungal strain are well characterized27. Given the complexity of C. albicans regulatory networks, which allow it to respond to diverse environmental cues28,29, we assessed the ability of 4-AQ derivatives to inhibit filamentation across seven media differing in their composition: N-acetyl glucosamine (GlnAc) medium, Lee’s medium, RPMI-1640 medium, Spider medium, Yeast-Peptone Sucrose (YPS) medium with and without uridine, and Synthetic Low Ammonium Dextrose (SLAD) medium30. This comprehensive approach enabled us to thoroughly evaluate the anti-virulence potential of the 4-AQ library under diverse conditions that trigger yeast-to-hyphae switch in C. albicans. With the aim to specifically identify compounds that inhibit filamentation without impairing fungal growth, we selected the all 4-AQ derivatives with MIC values ≥ 50 µM and tested them at a concentration of 10 µM (Fig. 1d). Additionally, several derivatives exhibiting antifungal activity were included in the analysis to further elucidate structure-activity relationships and their influence on filamentation inhibition.
Without treatment, C. albicans SC5314 formed dense hyphae and/or wrinkled colonies on the solid media after 3-5 days of incubation (Fig. 1e). On the other hand, each of the thirteen 4-AQ molecules tested blocked filament formation in SLAD medium, of which 10 derivatives acted in Spider medium and one derivative also acted in Lee and RPMI media, however, none molecule suppressed the development of hyphae in the presence of GlcNAc (Fig. 1d, e).
Based on these data and the hierarchical cluster analysis, the tested 4-AQ molecules and HHQ were clustered into four distinct groups according to their filamentation-inhibitory potency and activity patterns (Fig. 1d). Notably, Group III (molecule 6 and HHQ) and Group IV (molecules 12, 22, 23, and 24) comprised the most effective entities against C. albicans morphogenesis. These derivatives successfully inhibited the development of hyphae under nutrient conditions that C. albicans may encounter in the human body, such as glucose-enriched environments (e.g., oral cavity, bloodstream, and diabetic tissues) or sucrose-rich niches (e.g., transiently present in the mouth, stomach, and intestine), as well as regions limited in nitrogen and amino acids supply (e.g., vaginal mucosa and inflamed oral tissues). Additionally, derivatives 12, 22, 23, and 24 suppressed filamentation under embedding conditions simulated by YPS medium, found in the deep-seated infection microenvironments31,32,33,34,35. Interestingly, molecule 6, distinguished by its unique side-chain structure (Fig. 1a), was the only derivative capable of inhibiting filamentation in the amino acid-enriched conditions (RPMI medium), found in niches such as the bloodstream, saliva, and gastrointestinal tract. Given the metabolic plasticity and ability of C. albicans to adapt to diverse carbon and nitrogen sources which contributes significantly to its virulence and pathogenicity36, the activity of the 4-AQ series to inhibit filamentation under various conditions underscores the noteworthy anti-virulence potential of this class of molecules.
When analyzing the structure-anti-virulence activity relationship, we found that all tested 4-AQ molecules and HHQ blocked the formation of hyphae in SLAD medium, suggesting that the quinoline-like moiety may be responsible for the observed activity. The yeast-to-hyphae transition in C. albicans is known to be regulated by a complex network of interconnected biochemical pathways26,37. While specific pathways like Ras1-cAMP and Cek1-Cph1 are known to contribute to filamentation under nitrogen-limited (SLAD), glucose-free (Spider) and the embedding matrix environment conditions (YPS)26,31,37,38,39, the observed inhibition of filamentation of C. albicans in the different media with different stimuli suggests that 4-AQ derivatives may interfere with multiple morphogenetic pathways, reflecting the complexity of filamentation regulation under variable environmental conditions, or may act on some of the downstream central regulators of filamentation (i.e., EFG1, UME6, TEC1 and other transcription factors)40,41.
Interaction of 4-AQ compounds with nystatin in suppressing C. albicans filamentation
As the filamentous form of Candida albicans plays a crucial role in its pathogenicity, contributing to tissue invasion, immune evasion, and biofilm formation, we investigated the synergy between novel filamentation-inhibiting 4-aminoquinolines and nystatin, a polyene antifungal that has long been a cornerstone in the treatment of candidiasis, particularly of mucocutaneous and oropharyngeal infections. Such combination treatment is expected to lead to more effective treatment regimens, especially for resistant or recurrent infections. We selected 4-AQ derivatives 6 and 24 which successfully inhibited filamentation of C. albicans on the four different media, but also derivative 11 due to its strong anti-virulence activity on P. aeruginosa19.
The effect of the combination treatment on fungal filament formation was investigated in solid Spider and RPMI media and compared with the effect of the individual treatments and control (0.1% DMSO). Nystatin was administered at a dosage of 0.25–1 µM (corresponding to ¼×MIC − 1 × MIC) and each of the 4-AQ compounds was added at 1.25, 2.5 and 5 µM. The phenotypic changes were evaluated by filament length and filamentation density (Supplementary Fig. 1) and shown by the color-coded heat maps (Fig. 2a, c).
Inhibition of filamentation of C. albicans SC5314 in combined treatment with nystatin and 4-AQ molecules (6, 11 and 24) compared to the individual agents, evaluated in RPMI medium (a, b) and Spider medium (c, d). Color-based heat maps (a, c) show the categorized filamentation phenotypes after the 3-day treatments, assessed by the morphology of the fungal colonies (filament density and length, b, d) in comparison to the control (DMSO) as follows: no effect (very dense and long filaments developed; black boxes), weak inhibitory effect (lower filamentation density with long filaments; dark gray boxes), moderate inhibitory effect (short filaments developed; light gray boxes), good inhibitory effect (rare filaments developed; light green boxes), and complete inhibition (no filaments developed; green boxes).
Depending on the medium used, different interactions were observed between the tested 4-AQs and nystatin. In RPMI medium, nystatin reduced filament formation of C. albicans SC5314 only at 1×MIC, while 4-AQs hindered hyphae growth at 5 and 2.5 µM. However, the combination treatment was significantly more effective than individual agents and completely prevented filament formation even at ¼×MIC of nystatin in combination with 5 µM of 6 and 24 (Fig. 2a, b). In the Spider medium, nystatin also reduced filament growth only at 1×MIC (Fig. 2c, d), while 6, 11 and 24 completely prevented filaments at 1.25 µM, emphasizing their potent anti-virulence activity at a very low concentration. The combination of nystatin in ¼×MIC or ½×MIC with any concentration of 4-AQs tested was more effective than nystatin alone. However, weak antagonism was observed between 11 and 24 and the highest concentrations of nystatin, but not between 6 and nystatin (Fig. 2d). Taken together, these data suggest that the tested 4-AQ anti-virulence compounds have a synergistic interaction with the polyene antifungal and that the combination of their doses required for effective suppression of C. albicans filamentation may depend on environmental conditions.
The effect on C. albicans biofilm
The biofilm is one of the most important virulence factors contributing to the pathogenesis and drug resistance of C. albicans42. Therefore, compounds 6, 11, and 24 were further investigated for their anti-biofilm activity on C. albicans SC5314. We found that each of these molecules has the ability to inhibit biofilm formation (Fig. 3a) and disintegrate mature fungal biofilms (Fig. 3b) at doses below their MICs. Compound 11 appeared to be the most effective, with BFIC50 and BEC50 values of 10 µM and 15 µM, respectively (Table 1).
The molecules were administered at different doses and examined for the ability to a inhibit biofilm formation (at 6.25–50 µM) and b disintegrate 24-hour-old biofilm (25-100 µM) of C. albicans SC5314. Values are the average of a representative experiment performed in quadruplicate ± SD. Statistically significant differences between the treated groups and the control (0.1% DMSO) were determined using one-way ANOVA and the Bonferroni test (n.s. P > 0.5, *** P < 0.001). c Confocal microscopy images of the mixed biofilm of C. albicans (RFP-expressing, M137 strain) and P. aeruginosa PAO1 (GFP-expressing) formed in the presence of 0.1% DMSO (control) or 50 µM of 11 showed that the applied.
Given its efficacy against both C. albicans (this study) and P. aeruginosa biofilms (our previous data)19, 4-AQ derivative 11 was further tested against mixed biofilms of these two species. The mixed biofilms pose a significant health problem in clinical practice, as the common cooperative interactions between these two pathogens create a microenvironment that enhances their survival and resistance to host defenses. Confocal microscopy showed that treatment with 50 µM of compound 11 for 24 h significantly reduced the formation of a mixed biofilm (Fig. 3d), inhibiting the development of hyphae of C. albicans (red) and leaving it mainly in the yeast form within the mixed biofilm, while simultaneously reducing bacterial cells (green) adhesion and aggregation. These results highlight compound 11 as a promising candidate for combating fungal, bacterial, and mixed biofilms, which are critical for pathogenesis and drug resistance.
Liver and blood toxicity evaluation
Considering that the adverse side effects of clinical antifungals prevent their parenteral and long-term use (i.e., hemolysis and liver toxicity of polyenes and azoles)43, we next evaluated 6, 11, and 24 for the hematotoxicity in sheep red blood cells (sRBCs) and the hepatotoxicity using the reporter Tg(-2.8fabp10:EGFP) zebrafish line with fluorescently labeled liver.
The data obtained in these assays showed that neither 11 nor 24 were cytotoxic to sRBC (Fig. 4a, b) and caused no signs of liver toxicity (Fig. 4c, d) at concentrations up to 40–50 µM (no significant differences in hemolysis rate and liver area index between untreated and treated groups; P > 0.5, for both), suggesting that the tested 4-AQs have no side effects at the anti-biofilm concentrations and at the 5-10 times higher concentration at which filament formation was blocked. On the other hand, 6 at concentrations ≥10 µM caused red blood cell lysis (P > 0.001) and decreased liver area index in treated zebrafish embryos (P = 0.0283), suggesting that it is not suitable for systemic use and could possibly be considered a topical agent like HHQ and miconazole.
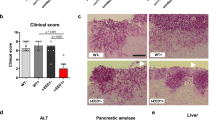
a The hemolytic activity tested on sheep red blood cells was compared with that of HHQ and miconazole, a clinical antifungal drug used as a positive control. b The hemolysis rate was determined in relation to the hemolysis of erythrocytes induced by 1% Triton X, which was arbitrarily set to 100%. Values are average of two independent experiment performed in triplicates ± SD. Statistically significant differences between the treated groups and control (0.1% DMSO) were determined using one-way ANOVA (***P < 0.001). c Hepatotoxicity was evaluated in transgenic Tg(-2,8fabp10:EGFP) zebrafish embryos with fluorescently labeled liver (n = 8) using the liver area index. d The morphology of the transgenic zebrafish embryos and their liver fluorescence after different treatments is shown. One-way ANOVA with the Bonferroni test was used to determine statistical significance by comparing fluorescence signal of the control sample with each treated group (*** P ≤ 0.001).
Assessing the 4-AQs activity in vivo against Candida albicans infections
To prove the efficacy of selected 4-AQ derivatives in vivo, we used the zebrafish infection model of lethal disseminated candidiasis44,45. Zebrafish infection models are widely used to study host interactions with numerous human pathogens46,47, including Candida species48. The small size and optical transparency of embryos provide an invaluable opportunity to simultaneously investigate the preclinical safety and efficacy of new antimicrobial agents, including virulence-interfering molecules, accelerating their development into new drugs or adjuvants46,47.
At first, we tested the efficacy of 6, 11, and 24 at a low (1.25 µM) and a high dose (10 µM), assessing their effect on fungal filamentation and the survival of infected embryos. Infection was established by injecting RFP-expressing C. albicans cells into the hindbrain of zebrafish embryos, an epithelium-surrounded structure that prevents the spread of the fungus. In untreated fish, a dense filamentous network invading the epithelium of the head developed in almost all embryos within 24 h post infection (hpi) (Supplementary Fig. 2a), leading to a 25% mortality rate, which increased to 65% by 96 hpi (Supplementary Fig. 1c). On the other hand, treatment with 4-AQ molecules successfully prevented C. albicans to form filaments and rescued the embryos from lethal infection. Fluorescence microscopy analysis showed that the yeasts-to-hyphae transition was suppressed even at low concentration of 1.25 µM, while no hyphae appeared in 80–100% of infected embryos treated with 10 µM AQs (Supplementary Fig. 1b). Compound 24 appeared to be the most effective, enabling total eradication of fungal infection (no RFP signal detected) and the 100% survival rate of infected embryos, while HHQ was the least effective and inhibited hyphal growth in only 33% of embryos at the same dosage of 10 µM.
Improvement of the efficacy of nystatin treatment against lethal disseminated candidiasis in vivo
Recognizing that combination treatment with anti-virulence agents may be an effective strategy to enhance the effect of clinical antifungal drugs49,50, we next investigated the efficacy of nystatin in combination with 11 or 24 in vivo, the two best anti-virulence 4-AQ candidates. We first showed that although treatment with nystatin alone increased the survival rate of infected embryos compared to untreated group (65–75% vs. 45%, P < 0.001) (Fig. 5a), it was only partially effective in eradicating aggressive fungal infection at doses that did not cause adverse effects (≤1 × MIC). The clumps of hyphae still occurred in 30-50% of treated embryos (Fig. 5b, c), indicating that higher doses of drug (≥2 × MIC) are required for completely successful treatment (elimination of infection). However, a recent study by Pavic et al. 51. showed that nystatin doses above 2 × MIC caused toxic side effects such as hepatotoxicity and inflammatory reactions, while lower doses eliminated C. albicans cells in only 45–50% of infected embryos.
a Filamentation of C. albicans M137 expressing RFP at 24 h post-treatment (hpt). b Proportion of embryos with/without visible hyphae in the hindbrain at 24 hpt, assessed by fluorescence microscopy (n = 20 embryos in each group). c Dose-dependent effect of nystatin, 4-AQ (11 and 24) and their combinations on the progress of C. albicans infection. Each data point represents the integrated red fluorescence intensity of a single alive zebrafish embryo at 24 hpt, and the signal in each group (8 embryos) is expressed as mean ± SD. Statistical significance was determined by χ2 test by comparing the distribution of embryos with/without hyphae in the control (DMSO-treated) and treated groups (*** P ≤ 0.001) as well as in the groups receiving nystatin and combination treatments (## P ≤ 0.01). Also, one-way ANOVA with the Bonferroni test was used to determine statistical significance by comparing fluorescence signal of the control (DMSO-treated) sample with each treated group (*** P ≤ 0.001), as well as between the nystatin-treated and the corresponding combination-treated group (## P ≤ 0.01). d the survival of the infected embryos treated with nystatin, 4-AQ and their combination compared to DMSO-treated embryos (control), as represented by Kaplan-Meier curve. Statistically significant differences in the survival between the treated and untreated groups (## P ≤ 0.01), as well as between the nystatin-treated groups with the corresponding combination treatment groups (## P ≤ 0.01) were determined using a log rank (Mantel-Cox) test.
Here, we demonstrated that the addition of 4-AQs significantly increased the efficacy of treatment with nystatin. After 24-h treatment, the infection was completely eliminated with nystatin and 24 (no RFP signal was detected) and markedly reduced (up to 14.7-fold) with nystatin plus 11 (Fig. 5a–c). The survival rate of C. albicans-infected embryos increased by up to 100% with combination treatments compared to the groups receiving nystatin alone (65–75%) or no treatment (45%) (P < 0.001 for both comparisons, Fig. 5d). These data indicate in vivo synergism between nystatin and 24 (at each combination dose) or 11 (at the higher doses tested), suggesting that combination treatment with 4-AQs may represent a new solution for the effective eradication of life-threatening fungal infections.
Altogether, our results demonstrate that the 4-AQ molecules originally developed to thwart the virulence mechanisms of P. aeruginosa, exhibit a previously unknown spectrum of anti-virulence activities reflected in their propensity to block filament formation, a key virulence trait of C. albicans, and to inhibit and disintegrate its biofilms. This discovery sheds light on the fact that a 4-AQ scaffold appears to be an important pharmacophore for inhibiting the development of hyphae under all conditions tested, while other parts of the molecule may enhance this anti-virulence activity in response to various stimuli to which C. albicans may be exposed during colonization of the different niches in the human body. The finding that the investigated 4-AQ derivatives effectively suppressed the pathogenicity of C. albicans, saved infected animals from a lethal fungal infection and significantly improved the efficacy of the clinical drug nystatin opens a new avenue and suggests a new strategy in the fight against candidiasis. Moreover, the extensive in vitro and in vivo data collected in this and our previous study19 have shown that the 4-AQ derivatives studied are true anti-virulence agents that can also inhibit the formation of mixed biofilms composed of two opportunistic human pathogens such as P. aeruginosa and C. albicans. These results emphasize that small molecules such as the 4-AQ derivatives studied can unlock the potential of existing therapies and increase the efficacy against polymicrobial infections. Further evaluation of the efficacy of other clinical antifungals (i.e., azoles, echinocandins, flucytosine) in combination with 4-AQ warrants to be fully explored.
The study and data also align with WHO and UN recommendations for a comprehensive review of non-traditional antimicrobials with novel mechanisms of action that go beyond conventional drug development pathway, particularly in the context of the growing resistance to existing antifungals and the limited number of new drug entities in development. Considering the relationship between chemical structure and bioactivity of the 4-AQ series, the data obtained in this study can serve as a blueprint for the development of new aminoquinolines with improved anti-virulence potency, and to direct our further efforts towards elucidating their mechanism of action through the various omics studies and gene expression analyzes in the relevant in vitro conditions and animal models of infection.
Methods
Chemicals
All chemicals including solvents (DMSO), buffers (MOPS), polyvinylpyrrolidone (PVP), XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide), MS-222 (tricaine), HHQ (heptyl-4-hydroxyquinoline), antifungals (nystatin, amphotericin B, itraconazole and miconazole), and microbiological media constituents were obtained from the Sigma (Munich, Germany). All chemicals were of reagent-grade quality or higher and used without further purification. The 4-aminoquinoline derivatives (4-AQ) examined in this study are part of the compound library synthetized and characterized in our previous study 52.
Fungal and bacterial strains and growth conditions
Candida albicans ATCC SC5341, C. albicans ATCC10231, C. glabrata ATCC2001 and Pseudomonas aeruginosa PAO1 NCTC 10332 used in the study were obtained from the respective reference strains collections. C. albicans strain M137 expressing red fluorescent protein (RFP) was provided by Prof. Bernhard Hube, Department of Microbial Pathogenicity Mechanisms, Hans Knoell Institute, Jena, Germany 53. C. albicans was grown in YPD or RPMI-1640 medium (with glutamine and phenol red, without bicarbonate) buffered with MOPS at 30 °C on a rotary shaker at 180 rpm. Bacterial strains, including P. aeruginosa PAO1 expressing GFP21, were grown in Luria Bertani (LB) broth at 37 °C on a rotary shaker at 180 rpm.
Antifungal susceptibility of planktonic cells
The minimum inhibitory concentrations (MIC) of 4-AQ derivatives, HHQ and three antifungals (nystatin, amphotericin B and itraconazole) were determined according to standard broth microdilution assays recommended by the EUCAST standard54. Stock solutions of all tested compounds and antifungals were prepared in DMSO. The highest tested concentration of 4-AQ derivatives and HHQ was 1 mM, while of nystatin, amphotericin B and itraconazole was 4 µM. Assay was performed in RPMI-1640 supplemented with 2% glucose in final concentration of C. albicans inoculums containing 2 × 105 colony forming units (CFU) per mL. The MIC value corresponds to the lowest concentration of tested compounds that inhibited the growth after 20 h at 37 °C. The experiment was performed in triplicate and repeated twice.
Inhibition of fungal filamentation
The anti-virulence potential of 4-AQ molecules was investigated under different growth conditions that induce filamentation of C. albicans cells, using solid media such as Spider33, Yeast extract Peptone Sucrose (YPS) with/without uridine (31, medium with N-acetyl glucosamine (GlcNAc)35, Lee medium32, SLAD medium30 and RPMI-164034. Experiments were conducted in 24-well microtiter plates and repeated twice. In brief, the overnight culture of C. albicans SC5314 cultures grown in YPD medium at 30 °C on a rotary shaker (180 rpm) were washed three times with sterile phosphate-buffered saline (PBS; pH 7.2), diluted to a concentration of 106 cells/mL, and inoculated (2.5 µL) onto the surface of solid medium containing the different concentration of the tested molecules. 4-AQ and HHQ were initially screen at the concentration of 10 µM, while the best selected compounds were also tested at the three additional concentrations (1.25, 2.5 and 5 µM). A medium inoculated with fungal cells without treatment served as a control for filament formation. The inoculated plates were incubated at 37 °C for 4–6 days and examined daily for filament formation. The individual fungal colonies were photographed with a stereomicroscope (Carl Zeiss™ Stemi 508 doc stereomicroscope, Germany), and analyzed for the presence of filaments. After microscopic inspection of the treated colonies, each applied molecule was binary classified as active (no filaments present) or inactive (filaments present) and hierarchically classified using Ward’s clustering method and Euclidean distance (IBM SPSS Statistics Version 21.0.0, Inc., Armonk, NY, US) and displayed as a heatmap.
Checkerboard filamentation assay
The effect of the combined treatment with nystatin and the selected 4-AQ molecules (6, 11, and 24) on the filamentation of C. albicans SC5314 was tested in checkerboard assay on Spider and RPMI-1640 media in 24-well microtiter plates. Nystatin was administered at concentrations of 1 × MIC and ½ × MIC and 4-AQ molecules at concentrations of 1.25, 2.5, and 5 µM. Treatments with nystatin or 4-AQs alone were also included. DMSO (0.1%) was used as a control. The fungal cell suspension for inoculation was prepared after sub-culturing the overnight culture (grown in YPD at 30 °C on a rotary shaker at 180 rpm) under the same conditions to a mean exponential phase (OD530 = 0.7–0.8); the culture was washed three times in PBS and adjusted to the optical density of OD530 = 1 in PBS. Each medium was inoculated with 2 µL of a previously prepared cells suspension. The fungal filaments were examined after 2, 4, and 6 days of incubation at 37 °C using a stereomicroscope (Carl Zeiss™ Stemi 508 doc stereomicroscope, Zeiss, Oberkochen, Germany). The effect on filament formation of C. albicans SC5314 was compared between the control (DMSO), the single and combination treatments. The experiment was performed three times.
Biofilm quantification assays
Biofilm quantification assays were performed in 96-well microtiter plate format (Sarstedt, Munich, Germany) in RPMI-1640 supplemented with 2% (w/v) glucose. Overnight culture of C. albicans SC5314 was diluted to 106 cell/mL and 100 µL was added to the wells. After 2 h of adhesion phase, supernatant was removed, and adherent cells were washed two times with sterile PBS. To address the effect on biofilm formation compounds in increasing concentrations or DMSO (0.1%, v/v) were applied in 100 µL RPMI-1640 and after 24 h biofilms were quantified using XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assay for cell metabolic activity detection. The extent of XTT reduction to a water-soluble orange formazan compounds, was measured in the supernatant of treated cells spectrophotometrically as previously described55. To address the effect of the compounds on C. albicans mature biofilms, after 2 h of adhesion phase, supernatant was removed, and adherent cells were washed two times with sterile PBS and allowed to form biofilms for 24 h in RPMI 1640 with 2% (w/v) glucose. After 24 h incubation, supernatant was removed and, after two washing steps with PBS, biofilms were treated with compounds or DMSO (0.1%, v/v) in 100 µL RPMI supplemented with glucose for additional 24 h. Remaining biofilms were quantified using XTT assay as described above.
Confocal microscopy
Overnight culture of C. albicans SC5314, strain M137 expressing RFP53 was diluted in RPMI 1640 medium to obtain 106 cells per mL and 2 mL were added per well of 6-well microtiter plates containing glass cover slips. Cells were left to adhere for 3 h at 37 °C. After removal of non-attached fungal cells, the adherent cells were washed once with PBS and 2 mL of diluted overnight culture of P. aeruginosa PAO1-GFP21 in RPMI 1640 (2 × 105 cells per mL) were added, together with 11 (final concentration 50 µM). The control samples contained DMSO instead of 11. After 24 h static incubation at 37 °C, biofilms were washed with PBS and analyzed by a Leica TCS SP8 confocal microscope and Leica Microsystems LAS AF-TCS SP8 software (Leica Microsystems).
In vitro toxicity
The in vitro toxicity of a series of 4-AQs was tested on sheep red blood cells (sRBCs) based on their hemolysis rate. Fresh cells were washed three times in PBS, diluted to a 4% suspension and exposed to the five different concentrations (5, 12.5, 25, 50, and 80 µM) of each tested molecule for 24 h at 37 °C. After centrifugation (2200 rpm, 5 min), the absorbance of the supernatants was measured at 450 nm using a Tecan Infinite 200 Pro Multiplate Reader (Tecan Group Ltd., Männedorf, Switzerland). Triton X-100 (1%) was used as a positive control. The percentage of hemolysis (hemolysis rate) was expressed in relation to the hemolysis caused by Triton X-100, which was arbitrarily set at 100%. Hemolysis rate upon 4-AQs treatment was compared with the rate caused by HHQ and miconazole, an antifungal agent for the topical treatment of fungal infections.
In vivo experiments in the zebrafish (Danio rerio) model
All experiments with zebrafish (Danio rerio) embryos were performed in accordance with the European Directive 2010/63/EU and the Ethical Guidelines for the Care and Use of Laboratory Animals of the Institute of Molecular Genetics and Genetic Engineering of the University of Belgrade. Wild-type (AB) zebrafish embryos, kindly provided by Dr. Ana Cvejić (Wellcome Trust Sanger Institute, Cambridge, UK), were reared to the adult stage in a temperature- and light-controlled zebrafish facility at 28 °C and a standard 14:10 h light-dark photoperiod. The fish were fed twice daily with commercial dry food (SDS200 and SDS300 granular food; Special Diet Services, Essex; UK and TetraMinTM flakes; Tetra Melle, Germany) and daily with Artemia nauplii.
In vivo toxicity assessment
The toxicity of a series of 4-AQ, HHQ and clinical drugs (nystatin, amphotericin B and itraconazole) and their combinations was evaluated in accordance with the general rules of the OECD guidelines for the testing of chemicals56. Embryos generated by pairwise mating were washed to remove debris and distributed 10 per well in 24-well plates containing 1 mL of E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2 and 0.33 mM MgSO4 in distilled water) and maintained at 28 °C. To evaluate acute (lethal) and developmental (teratogenic) toxicity, embryos were exposed to the seven different concentrations (1.25, 2.5, 5, 12.5, 25, 50, and 100 µM) of each 4-AQ and HHQ, as well as to the six different concentrations of clinical drugs (0.25, 0.5, 1, 1.25, 2, and 2.5 µM) at 6 hours post-fertilization (hpf), an early embryonic stage that ensures high sensitivity to the molecules tested. The tested compounds (DMSO stock) are directly applied in the E3 medium. Treated embryos were examined every day under a stereomicroscope (Carl Zeiss™ Stemi 508 doc Stereomicroscope, Germany) for the appearance of apical endpoints (Table S1) until 120 hpf. Dead embryos were collected and discarded every 24 h. DMSO (0.25%) and E3 medium were used as negative controls. Experiments were performed in triplicate with 20 embryos for each concentration. At 120 hpf, embryos were anesthetized by the addition of 0.1% (w/v) Tricaine solution (Sigma-Aldrich, St. Louis, MO), photographed, and killed by freezing at −20 °C for ≥24 h. In order to assess the safety of combination treatment based on nystatin and the best 4-AQ compounds, the 6-hpf staged embryos were co-exposed to nystatin (0.5 and 1 µM) and 11 and 24 (1.25, 2.5, and 5 µM).
To test the selected 4-AQ molecules (6, 11, and 24) for a possible hepatotoxic effect in vivo, the embryos of transgenic Tg(-2.8fabp10a:EGFP) zebrafish embryos with the fluorescently labeled liver57 were exposed to the sublethal doses of the tested compounds (6.25–50 µM of 11 and 24, 6.15–25 µM of 6, 1.25–5 µM of HHQ) from the 72-hpf stage (when the liver is fully functional and has started metabolic transformation) up to the 120-hpf stage. Experiment was performed twice, using 10 embryos per concentration. At the 120 hpf, four embryos were randomly selected and examined under a fluorescence microscope for liver fluorescence. Hepatotoxicity was determined by the change in liver area index compared to the control group (0.1% DMSO), calculated as the ratio between the liver area and the embryonic lateral area × 100%, as predictive parameters of hepatotoxicity described in the literature57,58. The liver area and lateral area were determined from fluoresce images using the ImageJ program (NIH, LOCI-UW, United States).
Antifungal efficacy evaluation of the selected 4-AQs in vivo
The antifungal activity of the selected 4-AQs and HHQ was investigated in vivo using the zebrafish model of lethal disseminated candidiasis according to a previously described method44 and compared to the efficacy of nystatin. The C. albicans strain M137 expressing red fluorescent protein (RFP)53 was used for the infection experiments. In brief, the fungal culture grown overnight in YPD broth on the rotary shaker (180 rpm) at 30 °C was sub-cultured under the same growth conditions at a ratio of 1:200 until a middle exponential phase (OD530 = 0.7–0.8) was reached. After centrifugation at 2400 × g for 10 min (centrifuge 5415D, Eppendorf, Hamburg, Germany) and washing three times with sterile PBS, the fungal cells were suspended in 2% polyvinylpyrrolidone (PVP) to a final concentration of 2 × 107 cells/mL.
Prior to the infection experiment, embryos at the 24 hpf stage were manually dechorionated. To establish localized infection, a volume of ~5 nL of the fungal cell suspension (containing 60–78 yeast cells) was microinjected through the otic vesicle into the hindbrain of anesthetized zebrafish embryos at the 34 hpf stage. After a recovery period of 1 hour at 28 °C, the live embryos were divided into a 24-well plate (10 embryos per well) and treated with the selected 4-AQs (1.25, 2.5, 5, and 10 µM), nystatin (0.5 and 1 µM) or their combination. Embryos injected with only 2% PVP served as a control group (mock). The effect of the applied treatments on filamentation of C. albicans SC5314 cells and the survival of infected embryos was monitored daily for 4 days post-infection (dpi).
Statistical analysis
The dose-dependent effect on erythrocyte lysis and liver area index was analyzed using ANOVA and the Bonferonni test. The difference in the proportion of embryos with/without hyphae between the treatment and control groups was analyzed using the X2-test, while the difference in fungal mass, as assessed by fluorescence intensity (determined by pixels), was analyzed using ANOVA and the Bonferonni test. Survival rates were analyzed using the Kaplan–Meier method, comparisons between curves were performed using the log-rank test, while analysis was performed using GraphPad Prism version 6.0. In all tests, the statistical significance was assumed for P values < 0.5.
Data availability
All data supporting the findings of this study are included in the article and its supplementary files and figures. Additional data are available from the corresponding authors upon request.
References
Bongomin, F., Gago, S., Oladele, R. O. & Denning, D. W. Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi 3, 57 (2017).
Tortorano, A. M. et al. Candidaemia in Europe: epidemiology and resistance. Int. J. Antimicrob. Agents 27, 359–366 (2006).
Kanj, S. S. et al. The battle against fungi: lessons in antifungal stewardship from COVID 19 times. Int. J. Antimicrob. Agents 62, 106846 (2023).
Coordination G, Alastruey-Izquierdo A, World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action (Organización Mundial de la Salud (OMS), 2022).
Howard, K. C., Dennis, E. K., Watt, D. S. & Garneau-Tsodikova, S. A comprehensive overview of the medicinal chemistry of antifungal drugs: perspectives and promise. Chem. Soc. Rev. 49, 2426–2480 (2020).
Perfect, J. R. The antifungal pipeline: a reality check. Nat. Rev. Drug Discov. 16, 603–616 (2017).
Nett, J. E. & Andes, D. R. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. North Am. 30, 51–83 (2016).
Arendrup, M. C. & Patterson, T. F. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 216, S445–S451 (2017).
Liu, N., Tu, J., Dong, G., Wang, Y. & Sheng, C. Emerging new targets for the treatment of resistant fungal infections. J. Med. Chem. 61, 5484–5511 (2018).
Kriegl, L., Egger, M., Boyer, J., Hoenigl, M. & Krause, R. New treatment options for critically important WHO fungal priority pathogens. Clin. Microb. Infect. S1198-743X(24)00118-6 (2024).
Vila, T. et al. Targeting Candida albicans filamentation for antifungal drug development. Virulence 8, 150–158 (2017).
Mayer, F. L., Wilson, D. & Hube, B. Candida albicans pathogenicity mechanisms. Virulence 4, 119–128 (2013).
Organization WH. Antibiotic Resistance: Multi-country Public Awareness Survey (World Health Organization, 2015).
Grainha, T., Jorge, P., Alves, D., Lopes, S. P. & Pereira MO. Unraveling pseudomonas aeruginosa and candida albicans communication in coinfection scenarios: insights through network analysis. Front. Cell. Infect. Microbiol. 10, 550505(2020).
Chotirmall, S. H. et al. Sputum Candida albicans presages FEV1 decline and hospital-treated exacerbations in cystic fibrosis. Chest 138, 1186–1195 (2010).
Azoulay, E. et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 129, 110–117 (2006).
Peleg, A. Y., Hogan, D. A. & Mylonakis, E. Medically important bacterial–fungal interactions. Nat. Rev. Microbiol. 8, 340–349 (2010).
Heeb, S. et al. Quinolones: from antibiotics to autoinducers. FEMS Microbiol. Rev. 35, 247–274 (2011).
Aleksic, I. et al. N-benzyl derivatives of long-chained 4-amino-7-chloro-quionolines as inhibitors of pyocyanin production in Pseudomonas aeruginosa. ACS Chem. Biol. 14, 2800–2809 (2019).
Reen, et al. The Pseudomonas quinolone signal (PQS), and its precursor HHQ, modulate interspecies and interkingdom behaviour. FEMS Microbiol. Ecol. 77, 413–428 (2011).
Senerovic, L. et al. Quinolines and quinolones as antibacterial, antifungal, anti-virulence, antiviral and anti-parasitic agents. Adv. Microbiol., Infect. Dis. Public Health 14, 37–69 (2020).
Reen, F. J. et al. Exploiting interkingdom interactions for development of small-molecule inhibitors of Candida albicans biofilm formation. Antimicrob. Agents Chemother. 60, 5894–5905 (2016).
Patton, E. E., Zon, L. I. & Langenau, D. M. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 20, 611–628 (2021).
Zon, L. I. & Peterson, R. T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 4, 35–44 (2005).
Cully M. Zebrafish earn their drug discovery stripes. Nat. Rev. Drug Discov. 811–814 (2019).
Noble, S. M., Gianetti, B. A. & Witchley, J. N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 15, 96–108 (2017).
Biswas, S., Dijck, P. V. & Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 71, 348–376 (2007).
Lee, Y., Hossain, S., MacAlpine, J., Robbins, N. & Cowen, L. E. Functional genomic analysis of Candida albicans protein kinases reveals modulators of morphogenesis in diverse environments. iScience 26, 106145 (2023).
Fox, E. P. & Nobile, C. J. A sticky situation. Transcription 3, 315–322 (2012).
Gimeno, C. J., Ljungdahl, P. O., Styles, C. A. & Fink, G. R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68, 1077–1090 (1992).
Kumamoto, C. A. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc. Natl Acad. Sci. 102, 5576–5581 (2005).
Lee, K. L., Buckley, H. R. & Campbell, C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13, 148–153 (1975).
Liu, H., Köhler, J. & Fink, G. R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266, 1723–1726 (1994).
Romo JA, et al. Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of Candida albicans filamentation. MBio. 8. https://doi.org/10.1128/mbio.01991-17 (2017).
Shepherd, M. G., Yin, C. Y., Ram, S. P. & Sullivan, P. A. Germ tube induction in Candida albicans. Can. J. Microbiol. 26, 21–26 (1980).
Miramón, P. & Lorenz, M. C. A feast for Candida: metabolic plasticity confers an edge for virulence. PLoS Pathog. 13, e1006144 (2017).
Sudbery, P. E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9, 737–748 (2011).
Brown Jr, D. H., Giusani, A. D., Chen, X. & Kumamoto, C. A. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 34, 651–662 (1999).
Vinces, M. D., Haas, C. & Kumamoto, C. A. Expression of the Candida albicans morphogenesis regulator gene CZF1 and its regulation by Efg1p and Czf1p. Eukaryot. Cell 5, 825–835 (2006).
Azadmanesh, J., Gowen, A. M., Creger, P. E., Schafer, N. D. & Blankenship, J. R. Filamentation involves two overlapping, but distinct, programs of filamentation in the pathogenic fungus Candida albicans. G3 Genes|Genomes|Genet. 7, 3797–3808 (2017).
Glazier, V. E. EFG1, everyone’s favorite gene in Candida albicans: a comprehensive literature review. Front. Cell Infect. Microbiol 12, 855229 (2022).
Ponde, N. O., Lortal, L., Ramage, G., Naglik, J. R. & Richardson, J. P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 47, 91–111 (2021).
Tverdek, F. P., Kofteridis, D. & Kontoyiannis, D. P. Antifungal agents and liver toxicity: a complex interaction. Expert Rev. Anti-infect. Ther. 14, 765–776 (2016).
Brothers, K. M., Wheeler, R. T. Non-invasive imaging of disseminated candidiasis in zebrafish larvae. JoVE (J. Vis. Exp). e4051 (2021).
Mallick, E. M. et al. Phenotypic plasticity regulates Candida albicans interactions and virulence in the vertebrate host. Front. Microbiol. 7, 780 (2016).
Gomes, M. C. & Mostowy, S. The case for modeling human infection in zebrafish. Trends Microbiol. 28, 10–18 (2020).
Torraca, V. & Mostowy, S. Zebrafish infection: from pathogenesis to cell biology. Trends Cell Biol. 28, 143–156 (2018).
Seman, B. G. et al. Yeast and filaments have specialized, independent activities in a zebrafish model of Candida albicans infection. Infect. Immunity. 86. https://doi.org/10.1128/iai.00415-00418 (2018).
Shapiro, R. S. & Gerstein, A. C. Powering up antiungal treatment: using small molecules to unlock the potential of existing therapies. Mbio 14, e01073–23 (2023).
Yang, S., Peng, X., Ren, B., Luo, Y. & Xu, X. Small molecule II-6s synergises with fluconazole against Candida albicans. Int. J. Antimicrob. Agents 62, 106820 (2023).
Pavic, A. et al. Polyenes in medium chain length polyhydroxyalkanoate (mcl-PHA) biopolymer microspheres with reduced toxicity and improved therapeutic effect against Candida infection in zebrafish model. Pharmaceutics 14, 696 (2022).
Aleksić, I. et al. Long-chain 4-aminoquinolines as quorum sensing inhibitors in Serratia marcescens and Pseudomonas aeruginosa. ACS Chem. Biol. 12, 1425–1434 (2017).
Fradin, C. et al. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 56, 397–415 (2005).
Giske, C. G., Turnidge, J., Cantón, R. & Kahlmeter, G. Update from the European committee on antimicrobial susceptibility testing (EUCAST). J. Clin. Microbiol. 60, e00276-21 (2022).
Peeters, E., Nelis, H. J. & Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72, 157–165 (2008).
OECD TN. 236: Fish embryo acute toxicity (FET) test. OECD Guidelines for the Testing of Chemicals, Section 2. 1–22 (OECD Publishing, 2013).
Her, G. M., Chiang, C. C., Chen, W. Y. & Wu, J. L. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio). FEBS Lett. 538, 125–133 (2003).
Zhang, Y. et al. A rapid assessment for predicting drug-induced hepatotoxicity using zebrafish. J. Pharmacol. Toxicol. Methods 84, 102–110 (2017).
Acknowledgements
This research is funded by Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grants No. 451-03-66/2024-03/200042 and 451-03-66/2024-03/200026). We thank Dr. Marija Schwirtlich from the Institute of Molecular Genetics and Genetic Engineering, University of Belgrade for assistance with confocal microscopy.
Author information
Authors and Affiliations
Contributions
A.P. and L.S. designed the study. A.P. performed high-content anti-virulence screening, interactions with drugs and in vivo toxicity and infection experiments. L.S. performed experiments related to biofilms. N.R., N.S. and I.M. performed the remaining in vitro experiments. D.O. synthesized the 4-AQ compounds. A.P. and L.S. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pavic, A., Radakovic, N., Moric, I. et al. Long-chain 4-aminoquinolines inhibit filamentation and increase efficacy of nystatin against Candida albicans infections in vivo. npj Biofilms Microbiomes 10, 146 (2024). https://doi.org/10.1038/s41522-024-00608-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41522-024-00608-3