Abstract
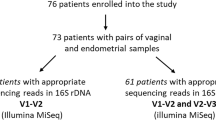
Miscarriage, the loss of a pregnancy before viability, can be sporadic or recurrent. Emerging evidence links miscarriage to specific microbiota compositions within the female reproductive tract (FRT). This systematic review aims to synthesise evidence on the association between sporadic and recurrent miscarriage and FRT microbiota composition, as assessed using metataxonomic profiling approaches. A systematic analysis of the 43 included studies, sampling the vaginal, cervical and endometrial microbiota supported an association between reduced Lactobacillus abundance and miscarriage, making it a potential target for therapeutic intervention. However, consistent changes in alpha and beta diversity were not observed and there was a lack of reproducibility for other compositional changes. This review also highlighted concerns about the significant bias introduced due to methodological variations and emphasises the need for future standardisation of microbial sampling, sequencing, and reporting to allow accurate comparison of results and to reduce research waste.
Similar content being viewed by others
Data availability
No new datasets were generated or analysed during the current study. The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
This is available from https://osf.io/9scwe/files/8na5e.
References
Quenby, S. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 397, 1658–1667 (2021).
Melo, P., Dhillon-Smith, R., Islam, M. A., Devall, A. & Coomarasamy, A. Genetic causes of sporadic and recurrent miscarriage. Fertil. Steril. 120, 940–944 (2023).
Bender Atik, R. et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum. Reprod. Open 2023, hoad002 (2023).
Ogasawara, M., Aoki, K., Okada, S. & Suzumori, K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil. Steril. 73, 300–304 (2000).
Berg, G. et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020).
Łaniewski, P., Ilhan, Z. E. & Herbst-Kralovetz, M. M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 17, 232–250 (2020).
Champer, M. et al. The role of the vaginal microbiome in gynaecological cancer. BJOG Int. J. Obstet. Gynaecol. 125, 309–315 (2018).
Mitra, A. et al. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 4, 58 (2016).
Toson, B., Simon, C.& Moreno, I. The endometrial microbiome and its impact on human conception. Int. J. Mol. Sci. 23, 485 (2022)
Gudnadottir, U. et al. The vaginal microbiome and the risk of preterm birth: a systematic review and network meta-analysis. Sci. Rep. 12, 7926 (2022).
Huang, C. et al. Meta-analysis reveals the vaginal microbiome is a better predictor of earlier than later preterm birth. BMC Biol. 21, 199 (2023).
Golob, J. L. et al. Microbiome preterm birth DREAM challenge: crowdsourcing machine learning approaches to advance preterm birth research. Cell Rep. Med. 5, 101350 (2024).
Gu, Y. et al. Gut and vaginal microbiomes in PCOS: implications for women’s health. Front. Endocrinol. 13, 808508 (2022).
Yoshikata, R. et al. Age-related changes, influencing factors, and crosstalk between vaginal and gut microbiota: a cross-sectional comparative study of pre- and postmenopausal women. J. Womens Health 31, 1763–1772 (2022).
Lebeer, S. et al. A citizen-science-enabled catalogue of the vaginal microbiome and associated factors. Nat. Microbiol. 8, 2183–2195 (2023).
Roachford, O. S. E., Alleyne, A. T. & Nelson, K. E. Insights into the vaginal microbiome in a diverse group of women of African, Asian and European ancestries. PeerJ 10, e14449 (2022).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. 108, 4680–4687 (2011).
Noormohammadi, M., Eslamian, G., Kazemi, S. N. & Rashidkhani, B. Association between dietary patterns and bacterial vaginosis: a case–control study. Sci. Rep. 12, 12199 (2022).
Noyes, N., Cho, K.-C., Ravel, J., Forney, L. J. & Abdo, Z. Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PLOS One 13, e0191625 (2018).
Lehtoranta, L., Ala-Jaakkola, R., Laitila, A. & Maukonen, J. Healthy vaginal microbiota and influence of probiotics across the female life span. Front. Microbiol. 13, 819958 (2022).
Song, S. D. et al. Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. mSphere 5, https://doi.org/10.1128/msphere.00593-20 (2020).
Chen, C. et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 8, 875 (2017).
van de Wijgert, J. & Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 168, 859–864 (2017).
Odendaal, J. et al. The endometrial microbiota and early pregnancy loss. Hum. Reprod. 39, 638–646 (2024).
Benner, M., Ferwerda, G., Joosten, I. & van der Molen, R. G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 24, 393–415 (2018).
D’Ippolito, S. et al. Endometrial microbes and microbiome: recent insights on the inflammatory and immune “players” of the human endometrium. Am. J. Reprod. Immunol. 80, e13065 (2018).
Al-Memar, M. et al. The association between vaginal bacterial composition and miscarriage: a nested case-control study. Bjog Int. J. Obstet. Gynaecol. 127, 264–274 (2020).
Chang, D. H. et al. Vaginal microbiota profiles of native Korean women and associations with high-risk pregnancy. J. Microbiol. Biotechnol. 30, 248–258 (2020).
Chen, S. et al. Vaginal atopobium is associated with spontaneous abortion in the first trimester: a prospective cohort study in China. Microbiol. Spectrum. 10, e0203921 (2022)
Fan, T. et al. The alteration and potential relationship of vaginal microbiota and chemokines for unexplained recurrent spontaneous abortion. Medicine 99, e23558 (2020).
Fernández, L. et al. Application of Ligilactobacillus salivarius CECT5713 to achieve term pregnancies in women with repetitive abortion or infertility of unknown origin by microbiological and immunological modulation of the vaginal ecosystem. Nutrients 13, 162 (2021)
Goncharov, A. E. et al. Features of microbiocenoses of various biotopes in women as a potential factor risk of miscarriage. Epidemiol. Vaccinal Prev. 20, 107–114 (2021)
Grewal, K. et al. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 20, 38 (2022)
Gryaznova, M. et al. Cervical and vaginal microbiomes in early miscarriages and ongoing pregnancy with and without dydrogesterone usage. Int. J. Mol. Sci. 24, 13836 (2023).
Guang, Y. et al. Systematic analysis of microbiota in pregnant Chinese women and its association with miscarriage. Ann. Transl. Med. 10, 1099 (2022)
Jiao, X. et al. Alteration of vaginal microbiota in patients with recurrent miscarriage. J. Obstet. Gynaecol. 42, 248–255 (2022).
Liu, X. et al. Association between vaginal microbiota and risk of early pregnancy miscarriage. Comp. Immunol. Microbiol Infect. Dis. 77, 101669 (2021).
Liu, F. T. et al. An altered microbiota in the lower and upper female reproductive tract of women with recurrent spontaneous abortion. Microbiol Spectr. 10, e0046222 (2022).
Masucci, L. et al. Celiac disease predisposition and genital tract microbiota in women affected by recurrent pregnancy loss. Nutrients 15, 221 (2023)
McClelland, R. S. et al. A prospective cohort study examining the association between the periconceptual vaginal microbiota and first-trimester miscarriage in Kenyan women. Paediatr. Perinat. Epidemiol. 38, 599–611 (2024).
Moreno, I. et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 215, 684–703 (2016).
Mori, R. et al. Cervicovaginal microbiome in patients with recurrent pregnancy loss. J. Reprod. Immunol. 157, 103944 (2023).
Peuranpää, P. et al. Female reproductive tract microbiota and recurrent pregnancy loss: a nested case-control study. Reprod. Biomed. Online 45, 1021–1031 (2022).
Severgnini, M. et al. A deep look at the vaginal environment during pregnancy and puerperium. Front. Cell Infect. Microbiol 12, 838405 (2022).
Shahid, M., Quinlivan, J. A., Peek, M., Castano-Rodriguez, N. & Mendz, G. L. Is there an association between the vaginal microbiome and first trimester miscarriage? A prospective observational study. J. Obstet. Gynaecol. Res. 48, 119–128 (2022).
Skafte-Holm, A. P. et al. The role of the vaginal microbiota, sexually transmitted infections, ureaplasmas and Mycoplasma hominis in spontaneous abortion: a prospective study in pregnant Danish women. https://doi.org/10.2139/ssrn.5194344 (2025).
Sun, D. et al. The association between vaginal microbiota disorders and early missed abortion: a prospective study. Acta Obstet. Gynecol. Scand. 101, 960–971 (2022).
Tan, J. F. et al. Correlation between Lactobacillus of vaginal microbiota and the pregnancy outcomes for patients experiencing recurrent miscarriage. Reproductive Sci. 32, 2729–2741 (2025).
van den Tweel, M. M. et al. Bacterial vaginosis in a subfertile population undergoing fertility treatments: a prospective cohort study. J. Assist. Reprod. Genet. 41, 441–450 (2024).
Wang, L. et al. Association between the vaginal and uterine microbiota and the risk of early embryonic arrest. Front. Microbiol. 14, 1137869 (2023).
Wang, L., Chen, Y., Wang, Q. & Wang, F. Microbial imbalances linked to early pregnancy loss: a comparative analysis of vaginal microbiota. J. Matern. Fetal Neonatal. Med. 38, 2496787 (2025).
Xu, L. F. et al. Vaginal microbiota diversity of patients with embryonic miscarriage by using 16S rDNA high-throughput sequencing. Int. J. Genom. 2020, 1764959 (2020)
Zhang, F. et al. Alteration of vaginal microbiota in patients with unexplained recurrent miscarriage. Exp. Ther. Med. 17, 3307–3316 (2019).
Zhao, F. et al. Characterization of vaginal microbiota in women with recurrent spontaneous abortion that can be modified by drug treatment. Front. Cell Infect. Microbiol. 11, 680643 (2021).
Gryaznova, M. V. et al. Pattern-recognition receptors and cervical microbiome in patients with early miscarriages. Int. J. Inflamm. 2024, 5320926 (2024).
Seo, S. S. et al. High prevalence of Leptotrichia amnionii, Atopobium vaginae, Sneathia sanguinegens, and factor 1 microbes and association of spontaneous abortion among Korean women. Biomed. Res. Int. 2017, 5435089 (2017).
Bai, S. et al. Investigating into microbiota in the uterine cavity of the unexplained recurrent pregnancy loss patients in early pregnancy. Placenta 152, 1–8 (2024).
Barinova, V. V., Kuznetsova, N. B., Bushtyreva, I. O., Dudurich, V. V. & Shatalov, A. E. Uterine microbiome and immunohistochemical markers of chronic endometritis in recurrent pregnancy loss. Obstet. Gynecol. 4, 84–94 (2022).
Bui, B. N. et al. The endometrial microbiota of women with or without a live birth within 12 months after a first failed IVF/ICSI cycle. Sci. Rep. 13, 3444 (2023).
Han, Y. Analysis of uterine microbiota in abortion and non-pregnant female based on high-throughput sequencing. J. Shanghai Jiaotong Univ. Med. Sci. 12, 165–169 (2019).
Liu, P. et al. The alteration of uterine microbiota participated in the activation of the decidual inflammatory response in early spontaneous abortion. PloS One 20, e0317595 (2025).
Moreno, I. V. F., Martinez, J. F., Codoner, F. M. & Ramon, D. Impact of the endometrial microbiome on uterine receptivity and pregnancy outcome. Reprod. Sci. 22, 224A (2015).
Moreno, I. et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome. 10, 1 (2022)
Shi, Y., Yamada, H., Sasagawa, Y., Tanimura, K. & Deguchi, M. Uterine endometrium microbiota and pregnancy outcome in women with recurrent pregnancy loss. J. Reprod. Immunol. 152, 103653 (2022).
Shu, J. J. et al. A potential role for the uterine microbiome in missed abortions. J. Biol. Regul. Homeost. Agents 36, 1055–1063 (2022).
Takimoto, K., Yamada, H., Shimada, S., Fukushi, Y. & Wada, S. Chronic endometritis and uterine endometrium microbiota in recurrent implantation failure and recurrent pregnancy loss. Biomedicines 11, 2391 (2023)
Vaughn, S. J., Moreno, I., Simon, C. & Lathi, R. B. Comparing the uterine microbiome in recurrent pregnancy loss to parous fertile controls. Fertil. Steril. 111, e17–e18 (2019).
Vomstein, K. et al. Uterine microbiota plasticity during the menstrual cycle: differences between healthy controls and patients with recurrent miscarriage or implantation failure. J. Reprod. Immunol. 151, 103634 (2022).
Wei, Q. et al. Impact of vaginal microecological differences on pregnancy outcomes and endometrial microbiota in frozen embryo transfer cycles. J. Assist. Reprod. Genet. 41, 929–938 (2024).
Mirzayi, C. et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat. Med. 27, 1885–1892 (2021).
O’Hanlon, D. E., Moench, T. R. & Cone, R. A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLOS One 8, e80074 (2013).
Vallor, A. C., Antonio, M. A., Hawes, S. E. & Hillier, S. L. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 184, 1431–1436 (2001).
Aroutcheva, A. et al. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 185, 375–379 (2001).
Molina, N. M. et al. Analysing endometrial microbiome: methodological considerations and recommendations for good practice. Hum. Reprod. 36, 859–879 (2021).
de Medeiros Garcia Torres, M. & Lanza, D. C. F. A standard pipeline for analyzing the endometrial microbiome. Reproductive Sci. 31, 2163–2173 (2024).
Chen, R. et al. Probiotics are a good choice for the treatment of bacterial vaginosis: a meta-analysis of randomized controlled trial. Reprod. Health 19, 137 (2022).
Ahrens, P. et al. Changes in the vaginal microbiota following antibiotic treatment for Mycoplasma genitalium, Chlamydia trachomatis and bacterial vaginosis. PloS One 15, e0236036 (2020).
Kadogami, D., Nakaoka, Y. & Morimoto, Y. Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod. Biol. 20, 307–314 (2020).
Thanaboonyawat, I., Pothisan, S., Petyim, S. & Laokirkkiat, P. Pregnancy outcomes after vaginal probiotic supplementation before frozen embryo transfer: a randomized controlled study. Sci. Rep. 13, 11892 (2023).
Kyono, K., Hashimoto, T., Kikuchi, S., Nagai, Y. & Sakuraba, Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: an analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 18, 72–82 (2019).
Wrønding, T. et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: a proof of concept case study. eClinicalMedicine 61, 102070 (2023)
Baud, A. et al. Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 13, 9061 (2023).
Leclair, C. M., Hart, A. E., Goetsch, M. F., Carpentier, H. & Jensen, J. T. Group B streptococcus: prevalence in a non-obstetric population. J. Low. Genit. Trac. Dis. 14, 162–166 (2010).
Vornhagen, J. et al. Group B streptococcus exploits vaginal epithelial exfoliation for ascending infection. J. Clin. Invest. 128, 1985–1999 (2018).
Onderdonk, A. B., Delaney, M. L. & Fichorova, R. N. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 29, 223–238 (2020).
Giakoumelou, S. et al. The role of infection in miscarriage. Hum. Reprod. Update 22, 116–133 (2016).
Liu, P., Lu, Y., Li, R. & Chen, X. Use of probiotic lactobacilli in the treatment of vaginal infections: In vitro and in vivo investigations. Front. Cell Infect. Microbiol. 13, 1153894 (2023).
Park, S. et al. Ureaplasma and Prevotella colonization with Lactobacillus abundance during pregnancy facilitates term birth. Sci. Rep. 12, 10148 (2022).
Poulsen, C. S., Kaas, R. S., Aarestrup, F. M. & Pamp, S. J. Standard sample storage conditions have an impact on inferred microbiome composition and antimicrobial resistance patterns. Microbiol. Spectr. 9, e0138721 (2021).
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org. (2025).
Kers, J. G. & Saccenti, E. The power of microbiome studies: some considerations on which alpha and beta metrics to use and how to report results. Front. Microbiol. 12, 796025 (2022).
Drevon, D., Fursa, S. R. & Malcolm, A. L. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav. Modif. 41, 323–339 (2017).
McGrath, S., Zhao, X., Steele, R., Thombs, B. D. & Benedetti, A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 29, 2520–2537 (2020).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014).
Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2000).
Acknowledgements
No direct funding was received for this work. N.B. and S.Q. are supported by Efficacy and Mechanism Evaluation Programme, an MRC and NIHR Partnership (Reference: 17/60/22). I.H. is supported by an MRC fellowship. J.O. is an NIHR Academic Clinical Lecturer. S.Q. and D.A.M. are supported by the Tommy’s National Centre for Miscarriage Research. D.A.M. is supported the March of Dimes.
Author information
Authors and Affiliations
Contributions
N.B. conceived the project. All authors contributed to the development of the protocol. N.B., I.H. and J.O. performed study selection and data extraction. N.B. performed the data analysis and completed the first draft of the manuscript. All authors (N.B., I.H., J.O., S.Q. and D.A.M.) reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.B., I.H., S.Q. and J.O. have no competing interests to declare. D.A.M. holds a patent for the use of Lactobacillus crispatus CTV-05 in the prevention of preterm birth (US63/151474).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Black, N., Henderson, I., Quenby, S. et al. Microbiota composition of the female reproductive tract and miscarriage: a systematic review and meta-analysis. npj Biofilms Microbiomes (2026). https://doi.org/10.1038/s41522-025-00901-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00901-9