Abstract
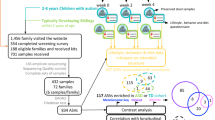
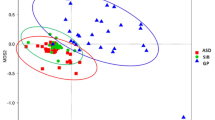
Anxiety and sensory hyperresponsiveness are common in children with autism spectrum disorder (ASD), but effective treatments are lacking. Targeting the microbiota-gut-brain axis is a promising strategy. This open-label pilot study evaluated SCM06, a novel synbiotic designed to target anxiety and sensory hyperresponsiveness, in 30 children with ASD (mean age 8.2 years, 22 males). We assessed symptom improvement, compliance, and safety, and collected stool samples for metagenomics and metabolomic analysis over 12 weeks. SCM06 was safe and well-tolerated, and significant improvements were observed in anxiety, sensory hyperresponsiveness, and abdominal pain. Following SCM06 treatment, increase in Bifidobacterium pseudocatenulatum was associated with improved functional abdominal pain (p = 0.0011, p_adj = 0.054), while the abundances of valeric acid and butyric acid increased (p_adj = 0.004 and p_adj = 0.072). Key microbial species, Coprococcus comes and Veillonella dispar, were candidate mediators of symptom improvements. Further randomised controlled trials are warranted to confirm its clinical efficacy.
Similar content being viewed by others
Data availability
Sequencing data were uploaded to NCBI under Bioproject PRJNA1274164 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1274164). Participant metadata cannot be made publicly available via repositories as outlined in the patient consent form to protect participant privacy. Requests for sharing metadata can be submitted with a written proposal to the corresponding author (Prof. Siew C. Ng) at siewchienng@cuhk.edu.hk.
References
Vasa, R. A., Keefer, A., McDonald, R. G., Hunsche, M. C. & Kerns, C. M. A scoping review of anxiety in young children with autism spectrum disorder. Autism Res. 13, 2038–2057 (2020).
La Buissonnière Ariza, V. et al. Predictors of suicidal thoughts in children with autism spectrum disorder and anxiety or obsessive-compulsive disorder: the unique contribution of externalizing behaviors. Child Psychiatry Hum. Dev. 53, 223–236 (2022).
Flouri, E., Midouhas, E., Charman, T. & Sarmadi, Z. Poverty and the growth of emotional and conduct problems in children with autism with and without comorbid ADHD. J. Autism Dev. Disord. 45, 2928–2938 (2015).
Aishworiya, R., Valica, T., Hagerman, R. & Restrepo, B. An update on psychopharmacological treatment of autism spectrum disorder. Neurotherapeutics 19, 248–262 (2022).
Green, S. A., Ben-Sasson, A., Soto, T. W. & Carter, A. S. Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: bidirectional effects across time. J. Autism Dev. Disord. 42, 1112–1119 (2012).
Lee, K. et al. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43, 2606–2614 (2018). 2018 43:13.
Guo, R., Chen, L. H., Xing, C. & Liu, T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br. J. Anaesth. 123, 637–654 (2019).
Morais, L. H., Schreiber, H. L. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255 (2020).
Umesawa, Y., Atsumi, T., Chakrabarty, M., Fukatsu, R. & Ide, M. GABA concentration in the left ventral premotor cortex associates with sensory hyper-responsiveness in autism spectrum disorders without intellectual disability. Front Neurosci. 14, 516744 (2020).
Uzunova, G., Pallanti, S. & Hollander, E. Excitatory/inhibitory imbalance in autism spectrum disorders: implications for interventions and therapeutics. World J. Biol. Psychiatry 17, 174–186 (2015).
Su, Q. et al. Multikingdom and functional gut microbiota markers for autism spectrum disorder. Nat. Microbiol. 2024, 1–12 (2024).
Soleimanpour, S., Abavisani, M., Khoshrou, A. & Sahebkar, A. Probiotics for autism spectrum disorder: an updated systematic review and meta-analysis of effects on symptoms. J. Psychiatr. Res. 179, 92–104 (2024).
Tan, Q. et al. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: a systematic review. Autism Res. 14, 1820–1836 (2021).
Lord, C. et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 399, 271–334 (2022).
Masi, A., DeMayo, M. M., Glozier, N. & Guastella, A. J. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci. Bull. 33, 183–193 (2017). 2017 33:2.
Kim, N. Y., Kim, S. K. & Ra, C. H. Evaluation of gamma-aminobutyric acid (GABA) production by Lactobacillus plantarum using two-step fermentation. Bioprocess Biosyst. Eng. 44, 2099–2108 (2021).
Turroni, F. et al. Bifidobacterium bifidum PRL2010 modulates the host innate immune response. Appl. Environ. Microbiol. 80, 730–740 (2014).
Uriot, O. et al. Streptococcus thermophilus: from yogurt starter to a new promising probiotic candidate? J. Funct. Foods 37, 74–89 (2017).
Yao, S., Zhao, Z., Wang, W. & Liu, X. Bifidobacterium Longum: protection against inflammatory bowel disease. J. Immunol. Res. 2021, 8030297 (2021).
Gau, S. S. F., Liu, L. T., Wu, Y. Y., Chiu, Y. N. & Tsai, W. C. Psychometric properties of the Chinese version of the Social Responsiveness Scale. Res. Autism Spectr. Disord. 7, 349–360 (2013).
Leung, P. W. L. et al. Test-retest reliability and criterion validity of the Chinese version of CBCL, TRF, and YSR. J. Child Psychol. Psychiatry 47, 970–973 (2006).
Chakraborty, P. et al. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism 25, 405–415 (2021).
Marler, S. et al. Association of rigid-compulsive behavior with functional constipation in autism spectrum disorder. J. Autism Dev. Disord (2017).
Dovgan, K., Gynegrowski, K. & Ferguson, B. J. Bidirectional relationship between internalizing symptoms and gastrointestinal problems in youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 53, 4488–4494 (2023).
Ho, I. et al. The distinctive clinical profiles of children with autism suffering from different subtypes of Rome IV functional gastrointestinal disorders. Autism Res. 0, 1–12 (2025).
Wong, O. W. H. et al. Disentangling the relationship of gut microbiota, functional gastrointestinal disorders and autism: a case–control study on prepubertal Chinese boys. Sci. Rep. 12, 1–13 (2022).
Drossman, D. A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology (2016).
Thapar, N. et al. Paediatric functional abdominal pain disorders. Nat. Rev. Dis. Prim. 6, 1–23 (2020).
Stern, E. K. & Brenner, D. M. Gut microbiota-based therapies for irritable Bowel syndrome. Clin. Transl. Gastroenterol. 9, e134 (2018).
Wong, O. W. H. et al. An elevated anxiety level among prepubertal autistic boys with non-treatment-seeking functional gastrointestinal disorders: a case–control study. Autism Res. 14, 2131–2142 (2021).
Wong, I. H. W. et al. The distinctive clinical profiles of children with autism suffering from different subtypes of Rome IV functional gastrointestinal disorders. Autism Res. (2025).
Louis, P. & Flint, H. J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41 (2017).
Rivière, A., Selak, M., Lantin, D., Leroy, F. & De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7, 206602 (2016).
Stilling, R. M. et al. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 99, 110–132 (2016).
Sharon, G. et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice in brief. Cell 177, 1600–1618.e17 (2019).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). 2015 18:7.
Moretti, M. et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav. Pharmacol. 22, 766–772 (2011).
Bonaz, B., Bazin, T. & Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 12, 336468 (2018).
Hoyles, L. et al. Microbiome–host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome 6, 1–13 (2018).
De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014).
Kang, J. H., Guo, X. D., Wang, Y. D. & Kang, X. W. Neuroprotective effects of N-acetylserotonin and its derivative. Neuroscience 517, 18–25 (2023).
Panula, P. & Nuutinen, S. The histaminergic network in the brain: basic organization and role in disease. Nat. Rev. Neurosci. 14, 472–487 (2013).
Baronio, D. et al. Histaminergic system in brain disorders: lessons from the translational approach and future perspectives. Ann. Gen. Psychiatry 13, 1–10 (2014).
Braniste, V. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6, (2014).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Wan, Y. et al. Fecal microbial marker panel for aiding diagnosis of autism spectrum disorders. Gut Microbes 16, 2418984 (2024).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). In Diagnostic and Statistical Manual of Mental Disorders (2013).
Rodgers, J. et al. Development of the anxiety scale for children with autism spectrum disorder (ASC-ASD). Autism Res. 9, 1205–1215 (2016).
Baranek, G. T., David, F. J., Poe, M. D., Stone, W. L. & Watson, L. R. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 47, 591–601 (2006).
Liu, J., Cheng, H. & Leung, P. W. L. The application of the preschool child behavior checklist and the caregiver-teacher report form to Mainland Chinese children: syndrome structure, gender differences, country effects, and inter-informant agreement. J. Abnorm Child Psychol. 39, 251–264 (2011).
Hyams, J. S. et al. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology (2016).
Velasco-Benítez, C. A., Oliveros, L. F. G., Moreno, L. M. R., Cuevas, J. R. T. & Saps, M. Diagnostic accuracy of the Rome IV Criteria for the Diagnosis of Functional Gastrointestinal Disorders in Children. J. Pediatr. Gastroenterol. Nutr. (2020).
Kwok, F. Y. Y., Ho, Y. Y. F., Chow, C. M., So, C. Y. N. & Leung, T. F. Assessment of nutrient intakes of picky-eating Chinese preschoolers using a modified food frequency questionnaire. World J. Pediatrics (2013).
National Health and Family Planning Commission of PRC. Chinese dietary reference intakes, WS/T 578. (2018).
Acknowledgements
The authors would like to thank the Child and Adolescent Psychiatric Team of Alice Ho Miu Ling Nethersole Hospital for their effort in recruiting participants for the present study. This study was supported by InnoHK, the Government of Hong Kong Special Administrative Region (FKLC, SCN); The D. H. Chen Foundation (FKLC, SCN); and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program (SCN).
Author information
Authors and Affiliations
Contributions
O.W.H.W., S.S.M.C., H.M.T., F.K.L.C., S.C.N. designed the study, O.W.H.W., S.S.M.C., F.Y.M.M., C.K.S.S., J.Y.L.C. were responsible for the recruitment of subject, conducting the study trial, clinical data and biological samples collection. O.W.H.W., Z.X., Q.S., M.Y.T.W., C.P.C. were responsible for handling the biological samples and laboratory work. O.W.H.W. and Z.X. were responsible for the clinical and bioinformatic data analysis and drafting the manuscript. S.S.M.C., W.T., H.M.T., F.K.L.C., S.C.N. provided significant intellectual contribution to the manuscript. All authors have read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.C.N. has served as an advisory board member for Pfizer, Ferring, Janssen and Abbvie and received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie and Takeda; has received research grants through her affiliated institutions from Olympus, Ferring and Abbvie; is a founder member, non-executive director, non-executive scientific advisor and shareholder of GenieBiome Ltd which is non-remunerative; is a shareholder of MicroSigX Diagnostic Holding Limited; is a founder member, non-executive Board Director, and non-executive scientific advisor of MicroSigX Biotech Diagnostic Limited, which is non-remunerative; and receives patent royalties through her affiliated institutions. F.K.L.C. serves as the Principal Investigator for the Faecal Microbiota Transplantation Service under the Hospital Authority (HA). He is a Board Director of EHealth Plus Digital Technology Ltd., an HA-owned subsidiary driving the eHealth+ programme to transform the Electronic Health Record Sharing System into a comprehensive digital healthcare platform and advance other IT initiatives within the eHealth ecosystem. Additionally, he is a Board Director of CUHK Medical Services Limited. He is a shareholder of GenieBiome Holdings Limited and the co-founder, non-executive Board Chairman, and non-executive Scientific Advisor of its wholly owned subsidiary, GenieBiome Ltd. He is also a shareholder of MicroSigX Diagnostic Holding Limited and the co-founder, non-executive Board Chairman, and non-executive Scientific Advisor of its wholly owned subsidiary, MicroSigX Biotech Diagnostic Limited. He also serves as a Director of the Hong Kong Investment Corporation Limited and a member of the Steering Committee for the RAISe+ Scheme under the Innovation and Technology Commission. Furthermore, he is the Co-Director of the Microbiota I-Center (MagIC) Ltd.ZX is named inventors of patent applications that cover the therapeutic and diagnostic use of microbiome and receive patent royalties through her affiliated institutions. H.M.T. is a named inventors of patent applications held by the CUHK and MagIC thatcover the therapeutic and diagnostic use of microbiome. O.W.H.W., H.M.T., F.K.L.C. and S.C.N. are named inventors of a patent application related to SCM06 for improving anxiety and sensory hyperresponsiveness in ASD children held by the CUHK, which is licensed exclusively to GenieBiome Ltd. The other authors do not have a competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong, O.W.H., Xu, Z., Chan, S.S.M. et al. A novel synbiotic (SCM06) for anxiety and sensory hyperresponsiveness in children with autism spectrum disorder: an open-label pilot study. npj Biofilms Microbiomes (2026). https://doi.org/10.1038/s41522-025-00902-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00902-8