Abstract
Association of stromal tumor-infiltrating lymphocytes (sTILs) with survival outcomes among patients with metastatic breast cancer (MBC) remains unclear. The primary objective was to evaluate the association of sTILs with progression-free survival in randomized phase III trial CALGB 40502. sTILs were associated with progression-free and overall survival in chemotherapy-treated MBC when controlling for treatment arm; however, this effect did not remain significant after additional adjustment for hormone receptor status. CALGB is now part of the Alliance for Clinical Trials in Oncology. Trial Registration: ClinicalTrials.gov: NCT00785291.
Similar content being viewed by others
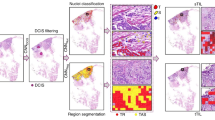
Quantification of stromal tumor-infiltrating lymphocytes (sTILs) via hematoxylin and eosin (H&E) stained slides using established approaches1 is an effective surrogate for host anti-tumor immunity2. sTILs are prognostic and associated with response to neoadjuvant chemotherapy (NAC) in primary triple negative breast cancer (TNBC) and HER2+ breast cancer3,4,5,6,7,8,9 yet their role in metastatic breast cancer (MBC) is less well defined. Microtubule-targeting agents are a mainstay of MBC treatment and Cancer and Leukemia Group B (CALGB) 40502 (Alliance) was a randomized phase III trial of 799 patients treated in the first-line setting, comparing microtubule-targeting chemotherapies: nab-paclitaxel, ixabepilone, or paclitaxel, with or without bevacizumab10. In this non-protocol-specified analysis, we hypothesized that sTILs quantity is significantly associated with progression-free survival (PFS) in MBC patients receiving first-line chemotherapy. In the primary analyses, sTILs were evaluated as <5% (low) versus ≥5% (high) based on prior analyses in MBC establishing 5% as a standard threshold11,12,13,14, with sensitivity analyses incorporating sTILs as a continuous variable.
Stromal TILs are lower in distant metastatic sites
sTILs distribution was skewed to low sTILs, with 373/582 (64.1%) with sTILs <5% and 155/582 (26.6%) with sTILs ≥5% (Fig. 1B). 68/582 (11.7%) samples demonstrated sTIL ≥50%, frequently considered ‘lymphocyte predominant’. Overall, samples from primary sites had higher average sTILs relative to locoregional recurrence or metastatic sites (mean 13.3% vs. 8.4%, respectively; Wilcoxon–Mann–Whitney p = 3e-4; Fig. 1C) and metastatic sites had the lowest mean sTILs (primary breast 12.7%, primary LN 22.9%, LRR 13.4%, distant metastasis 6.5%; Kruskal–Wallis p = 1e-5; Fig. 1D). Among distant metastatic sites, mean sTILs ranged from 1.3% bone (least) to 9.5% lung (greatest non-LN), and 20.4% LN (Kruskal–Wallis p = 1.4e-5; Fig. 1E). Of note, TILs in LN tissue is less reliable, impacted by presence of native nodal lymphocytes.
A CONSORT diagram. B Distribution of stromal TILs across all evaluable slides (n = 582). C Stromal TILs in combined primary samples (primary breast or lymph node) versus combined recurrent/metastatic (locoregional recurrence or distant metastatic sites). D Stromal TILs in primary breast versus primary lymph node versus locoregional recurrence versus distant metastatic sites. E Stromal TILs in distant metastatic sites. For C–E, box indicates 25th–75th percentile with median as center line and whiskers indicates 95th percentile above/below median. Mean value indicated below each variable. F Stromal TILs in paired primary breast cancer:metastasis or locoregional recurrence (LRR) within individual patients.
Evaluable slides from both primary and LRR or metastasis within the same patient were available for 100 unique patients. Among paired samples, primary tumors had significantly higher sTILs (mean 10.5%) relative to LRR or distant metastasis (mean 7.7%; Wilcoxon rank sum p = 0.008; Fig. 1F). Sensitivity analyses only evaluating primary:distant metastasis pairs demonstrated a trend that remained statistically significant (mean sTILs primary 7.6% vs. distant metastasis 6.3%; p = 0.011; data not shown).
Stromal TILs and association with survival outcomes
For all survival analyses, one slide per individual with the highest sTILs value was included for analyses (recurrent or metastatic site - 77/443, 17.4%; primary tumor – 366/443, 82.6%). Most patients had maximum sTILs <5% (259/443; 58.5%), with no significant difference in distribution by treatment arm, age, race, BMI, or inferred menopausal status (age > 55) (Table 1). There was a significantly greater proportion of high sTILs (≥5%) among hormone receptor-negative patients (73/114; 64.0%) than hormone receptor-positive patients (111/328; 33.8%). DFI (time from completion of adjuvant therapy to metastatic diagnosis) varied with greater proportion of de novo MBC (35/51; 68.6%) and DFI < 1 year (58/85; 68.2%) among low sTILs (<5%). Covariate data was missing for one individual.
For the primary objective, Cox proportional hazard model of sTILs low (versus high) was significantly associated with worse PFS (HR 1.34; 95% CI 1.1–1.63, p = 0.004) and OS (HR 1.32; 95% CI 1.07–1.63, p = 0.009; Table 2) when controlling for treatment arm. When controlling for both treatment arm and hormone receptor status, association of sTILs low versus high demonstrated similar trends but did not reach statistical significance for PFS (HR 1.2; 95% CI 0.97–1.47, p = 0.09) or OS (HR 1.14; 95% CI 0.91–1.43, p = 0.2; Table 2). When controlling for treatment arm, hormone receptor status, and clinicopathologic variables, association of sTILs low versus high demonstrated similar trends but further attenuated association for PFS (HR 1.17; 95% CI 0.94–1.44, p = 0.20) or OS (HR 1.06; 95% CI 0.84–1.34, p = 0.6; Table 2). There was no significant interaction between sTILs and treatment arm (all p-interaction >0.05). Sensitivity analyses among only breast primary samples with sTILs low vs. high and as a continuous variable and, separately, only distant metastases demonstrated that when controlling for treatment arm or both treatment arm and hormone receptor status, association of sTILs low versus high showed similar trends to the primary analyses but did not reach significance for PFS or OS (Supplementary Tables 1–3).
This study is the first trial to our knowledge to examine the association of sTILs with outcomes in MBC among patients receiving chemotherapy. Currently, there is not a reliable biomarker of immune activation that is applied clinically across centers; however, sTILs can readily be determined from routine H&E slide/image and, for TNBC and HER2+ breast cancers, appears to be consistently associated with response to NAC and prognosis among primary breast cancers. By evaluating primary:metastasis pairs from the same patient, we can see a consistent, significant decline in average sTILs. This difference varied patient-to-patient and also could be impacted by site of metastasis evaluated, given differences in sTIL amount, as has been seen in other studies15,16.
In this study, we found sTILs were associated with PFS in the metastatic setting, but that association was attenuated after controlling for HR status. This calls into question the robustness of the association and also in part reflects variations in sTILs between HR-negative and -positive tumors. Based on these findings, immune checkpoint inhibitor therapy is rational in mTNBC patients with pre-existing TIL12,13, yet the clinical challenge remains how to increase TIL/anti-tumor immunity in the metastatic setting. Given the extensive literature supporting association of sTILs with outcome and/or treatment response in primary3,4,5,6,7,8,9 and metastatic12,13,14 settings, a rational next step is design of prospective trials incorporating sTILs in patient stratification or treatment determination.
Limitations of this study include: sTILs enumeration on a mix of primary tumors, primary LN, LRR, and distant metastases adds heterogeneity to the evaluable data. Given significantly lower sTILs in LRR/metastatic specimens compared to primary, dedicated analyses of sTILs and PFS using only distant metastases in non-HR + MBC patients is needed.
In conclusion, immune activation measured by sTILs is significantly lower in metastatic than primary breast cancers and varies by metastatic site. We demonstrated that sTILs were associated with PFS and OS in chemotherapy-treated MBC, but the association of sTILs with outcome did not persist after controlling for hormone receptor status.
Methods
Study population
Clinical data were locked as of March 31, 2021. Of 788 patients receiving treatment on CALGB 40502, 690 H&E slides from 484 unique patients (62.7%) were submitted (Fig. 1A). Relative to the overall intention-to-treat population, patients with available tissue were balanced across arms and baseline characteristics (Table 1). None of the analyzed patients had HER2-positive breast cancer, which comprised only 2% of the CALGB 40502 study population. Protocol-specific written informed consent was obtained from participants and protocol approved by the National Cancer Institute’s Institutional Review Board. The informed consent document complies with federal and institutional guidelines, including the Declaration of Helsinki, for collection and use of data and samples. Overall, 582 slides were evaluable from 443 unique patients: 390 primary breast cancer, 26 primary lymph node (LN), 45 locoregional recurrence (LRR), 121 distant metastasis (Fig. 1A).
Tumor-infiltrating lymphocyte enumeration
TILs were enumerated in accordance with International TILs Working Group methods1,17. Briefly, one section (4–5 μm, magnification ×200–400) per sample was evaluated and TILs were reported for the stromal compartment (percent stromal TILs by study pathologist, RS). TILs were evaluated within the borders of the invasive tumor on full sections (preferred) or core biopsies. All analyses study used stromal TILs as the predefined TIL biomarker. As noted above, for the primary endpoint, sTILs were evaluated as <5% (low) versus ≥5% (high) based few existing studies11,12,13,14, with sensitivity analyses incorporating sTILs as a continuous variable.
Statistical analyses
The association between sTILs low/high and baseline characteristics of patients with evaluable sTILs was examined using two-sample t-test or rank sum tests for continuous variables and chi-squared test or Fisher’s exact test for categorical variables. Cox regression models were based on TIL-evaluable cohort for endpoints of PFS and OS to test the prognostic value of TILs, adjusting for treatment arm alone, with hormone receptor status, or with hormone receptor status plus body mass index (BMI), race, age at diagnosis, and disease-free interval (DFI). Proportional hazards assumptions were verified with Schoenfeld residuals. Data collection and statistical analyses were conducted by Alliance Statistics and Data Management Center.
Data availability
De-identified digital H&E images are available through data access request to the Alliance Standardized Translational Omics Resource (A-STOR). De-identified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data are not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that may have implications for the project, and ensuring that any transfer is in compliance with the IRB. The investigator will be required to sign a data release form prior to transfer.
Code availability
Statistical analyses were performed with SAS v9.4. Figures were generated with R v3.5.1.
References
Salgado, R., Loi, S., Denkert, C. International Immuno-Oncology Biomarker Working Group on Breast Cancer. (2023). www.tilsinbreastcancer.org.
Ali, H. R. et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 25, 1536–1543 (2014).
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13, 228–241 (2016).
Mao, Y. et al. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One 9, e115103 (2014).
Salgado, R. et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 1, 448–454 (2015).
Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28, 105–113 (2010).
Loi, S. et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann. Oncol. 25, 1544–1550 (2014).
Loi, S. et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 31, 860–867 (2013).
Liu, S. et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 16, 432 (2014).
Rugo, H. S. et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J. Clin. Oncol. 33, 2361–2369 (2015).
Kos, Z. et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 6, 17 (2020).
Loi, S. et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann. Oncol. 28, v608 (2017).
Loi, S. et al. Abstract PD5-03: relationship between tumor-infiltrating lymphocytes (TILs) and outcomes in the KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treated metastatic triple-negative breast cancer (mTNBC). Cancer Res. 80, PD5-03–PD05-03 (2020).
Emens, L. A. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 21, 1283–1295 (2020).
Richard, F. et al. Characterization of stromal tumor-infiltrating lymphocytes and genomic alterations in metastatic lobular breast cancer. Clin. Cancer Res. 26, 6254–6265 (2020).
Hilbers, F. et al. Characterization of the immune microenvironment in matched primary and metastatic breast cancer lesions from the AURORA study: BIG 14-01. J. Clin. Oncol. 41, 1009–1009 (2023).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2015).
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, and U24CA196171 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA233329, UG1CA233331, UG1CA233339, and UG1CA233373; Breast Cancer Research Foundation (D.G.S.), and the Alliance Foundation (D.G.S.). https://acknowledgments.alliancefound.org. Also supported in part by Bristol-Meyers Squibb (Abraxis/Celgene). NCI was involved in the design and conduct of the study and in review of the manuscript. Funders were not involved in collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to acknowledge Catherine Carson, Celia Garr, Katherine Tyson, and Ashley Little for clinical support making this research possible. This work was presented in part as an oral presentation as part of the Dr. Bernard Fisher Memorial Annual Clinical-Science Symposium at the 2023 Annual Meeting of the American Society of Clinical Oncology; June 1-6, 2023; Chicago, Illinois.
Author information
Authors and Affiliations
Contributions
Conception: D.G.S., R.S., L.A.C., A.H.P., H.S.R.; Data acquisition: D.G.S., M.V., K.G., M.W., Y.W., W.F.S., C.P., L.A.C., A.H.P., H.S.R.; Data analysis: D.G.S., R.S., O.S., K.B., H.S.R.; Manuscript first draft: D.G.S., R.S., H.S.R.; Manuscript review and approval: All authors.
Corresponding author
Ethics declarations
Competing interests
Authors have no conflicts of interest related to the current analyses. S.L. serves as a member of the Editorial Board of npj Breast Cancer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Stover, D.G., Salgado, R., Savenkov, O. et al. Association between tumor-infiltrating lymphocytes and survival in patients with metastatic breast cancer receiving first-line chemotherapy: analysis of CALGB 40502. npj Breast Cancer 10, 75 (2024). https://doi.org/10.1038/s41523-024-00683-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-024-00683-x
This article is cited by
-
Prognostic impact of tumor-infiltrating lymphocytes in HER2+ metastatic breast cancer receiving first-line treatment
npj Breast Cancer (2025)
-
Clinical utility of tumor-infiltrating lymphocyte evaluation by two different methods in breast cancer patients treated with neoadjuvant chemotherapy
Breast Cancer (2025)



