Abstract
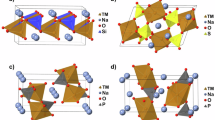
High-entropy layered oxides are promising sodium-ion battery (SIB) cathodes, yet the fundamental role of conformational entropy in stacking phase preference remains unclear. Here, we combine density functional theory (DFT), ab initio molecular dynamics (AIMD), and a fine-tuned CHGNet machine-learning interatomic potential (MLIP) to investigate representative high-entropy (Na0.8Ni0.2Fe0.2Co0.2Mn0.2Ti0.2O2) and low-entropy (Na0.8Mn0.6Co0.4O2) layered oxides in both O3 and P2 phases. A three-stage Monte Carlo sampling strategy was developed to explore transition-metal arrangements, Na/vacancy distributions, and representative low-energy conformations. The fine-tuned CHGNet achieved near-DFT accuracy while enabling large-scale sampling at orders of magnitude lower cost. Our analyses reveal that high-entropy oxides exhibit stronger Na–TMO2 interactions, broader O–TM bond length distributions, and smaller interlayer distance ratios compared with their low-entropy counterparts. These structural features favor O3-phase stabilization in cases where conventional ionic-potential descriptors are insufficient to clearly distinguish between O3- and P2-type layered oxides. Bond-length analyses further indicate that Jahn–Teller distortions in Mn are mitigated in high-entropy oxides, contributing to enhanced structural stability. This study establishes conformational entropy as a decisive factor, alongside Na ionic and cationic potentials, in governing stacking phase stability, and highlights the power of MLIP-accelerated modeling for exploring high-entropy materials and guiding the rational design of next-generation SIB cathodes.
Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available in https://github.com/KrisZhongLunLi/CHGNet-based-MC-sampling-toolkit.
References
Goodenough, J. B. & Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Cheng, X.-B., Zhang, R., Zhao, C.-Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Masias, A., Marcicki, J. & Paxton, W. A. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 6, 621–630 (2021).
Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 3, 1063–1069 (2017).
Tong, D. et al. Geophysical constraints on the reliability of solar and wind power worldwide. Nat. Commun. 12, 6146 (2021).
Killer, M., Farrokhseresht, M. & Paterakis, N. G. Implementation of large-scale Li-ion battery energy storage systems within the EMEA region. Appl. Energy 260, 114166 (2020).
Zhu, Z. et al. Rechargeable batteries for grid scale energy storage. Chem. Rev. 122, 16610–16751 (2022).
Feng, X. et al. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 10, 246–267 (2018).
Liu, B. et al. Safety issues caused by internal short circuits in lithium-ion batteries. J. Mater. Chem. A 6, 21475–21484 (2018).
Liu, X. et al. Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2, 2047–2064 (2018).
Xu, H., Chen, H. & Gao, C. Advanced graphene materials for sodium/potassium/aluminum-ion batteries. ACS Mater. Lett. 3, 1221–1237 (2021).
Balaram, V., Santosh, M., Satyanarayanan, M., Srinivas, N. & Gupta, H. Lithium: A review of applications, occurrence, exploration, extraction, recycling, analysis, and environmental impact. Geosci. Front. 15, 101868 (2024).
Yao, A., Benson, S. M. & Chueh, W. C. Critically assessing sodium-ion technology roadmaps and scenarios for techno-economic competitiveness against lithium-ion batteries. Nat. Energy 10, 404–416 (2025).
Wang, C.-Y. et al. Lithium-ion battery structure that self-heats at low temperatures. Nature 529, 515–518 (2016).
Ji, Y., Zhang, Y. & Wang, C.-Y. Li-ion cell operation at low temperatures. J. Electrochem. Soc. 160, A636 (2013).
Qahtan, T. F., Alade, I. O., AlArjani, A. & Rahaman, M. S. Advancements in sodium-ion batteries: An in-depth scientometric review. J. Energy Storage 131, 117490 (2025).
Priyadarshi, N. in Water Encyclopedia 551–553.
Abraham, K. M. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 5, 3544–3547 (2020).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Li, M. et al. Low-temperature performance of Na-ion batteries. Carbon Energy 6, e546 (2024).
Wang, K. et al. Recent advances in Mn-rich layered materials for sodium-ion batteries. Adv. Funct. Mater. 33, 2212607 (2023).
Kim, H.-J. et al. Synergetic impact of dual substitution on anionic–Cationic activity of P2-type sodium manganese oxide. Energy Storage Mater. 66, 103224 (2024).
Voronina, N. et al. Synergetic lattice and surface engineering: stable high-voltage cycle performance in P3-type layered manganese oxide. Adv. Energy Mater. n/a, 2501823.
Delmas, C., Fouassier, C. & Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C. 99, 81–85 (1980).
Voronina, N. et al. A new approach to stable cationic and anionic redox activity in O3-layered cathode for sodium-ion batteries. Adv. Energy Mater. 11, 2100901 (2021).
Voronina, N. et al. Electronic structure engineering of honeycomb layered cathode material for sodium-ion batteries. Adv. Energy Mater. 11, 2003399 (2021).
Ma, X. et al. Structural and electrochemical progress of O3-type layered oxide cathodes for Na-ion batteries. Nanoscale 15, 14737–14753 (2023).
Oh, S.-M. et al. High capacity O3-Type Na[Li0.05(Ni0.25Fe0.25Mn0.5)0.95]O2 cathode for sodium ion batteries. Chem. Mater. 26, 6165–6171 (2014).
Cho, M.-J. et al. Theoretical and Experimental Optimization of P2-Type Sodium-Ion Battery Cathodes via Li, Mg, and Ni Co-Doping: A Path to Enhanced Capacity and Stability. Adv. Energy Mater. 15, 2405112 (2025).
Yang, L. et al. Structural Aspects of P2-Type Na0.67Mn0.6Ni0.2Li0.2O2 (MNL) Stabilization by Lithium Defects as a Cathode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 31, 2102939 (2021).
Anang, D. A. et al. O3-type layer-structured Na0.8[Ni1/5Fe1/5Co1/5Mn1/5Ti1/5]O2 as long life and high power cathode material for sodium-ion batteries. Ceram. Int. 45, 23164–23171 (2019).
Liu, Z. et al. Achieving a Deeply Desodiated Stabilized Cathode Material by the High Entropy Strategy for Sodium-ion Batteries. Angew. Chem. Int. Ed. 63, e202405620 (2024).
Zeng, Z. et al. High-entropy O3-type cathode enabling low-temperature performance for sodium-ion batteries. Nano Energy 128, 109813 (2024).
Wang, X.-Z. et al. Fast Na+ Kinetics and Suppressed Voltage Hysteresis Enabled by a High-Entropy Strategy for Sodium Oxide Cathodes. Adv. Mater. 36, 2312300 (2024).
Urban, A., Seo, D.-H. & Ceder, G. Computational understanding of Li-ion batteries. npj Comput Mater. 2, 16002 (2016).
Wu, L.-T., Hwang, B. J. & Jiang, J.-C. Combined machine learning and computational protocols to predict electrolyte behavior and SEI formation in Li-metal batteries. Chem. Eng. J. 515, 163801 (2025).
Ito, D., Jang, S.-H., Ando, H., Momma, T. & Tateyama, Y. Dissimilar Diffusion Mechanisms of Li+, Na+, and K+ Ions in Anhydrous Fe-Based Prussian Blue Cathode. J. Am. Chem. Soc. 147, 25441–25453 (2025).
Wu, L.-T., Zhan, Y.-T., Chiu, Y.-C., Hwang, B. J. & Jiang, J.-C. Multifunctional Zwitterionic Self-Healing Polymer Electrolytes for Anode-Free Lithium-Metal Batteries: a Computational Perspective. Small 21, 2503382 (2025).
Sasaki, R., Gao, B., Hitosugi, T. & Tateyama, Y. Nonequilibrium molecular dynamics for accelerated computation of ion–ion correlated conductivity beyond Nernst–Einstein limitation. npj Comput. Mater. 9, 48 (2023).
Ko, S. et al. Electrode potential influences the reversibility of lithium-metal anodes. Nat. Energy 7, 1217–1224 (2022).
Takenaka, N., Bouibes, A., Yamada, Y., Nagaoka, M. & Yamada, A. Frontiers in Theoretical Analysis of Solid Electrolyte Interphase Formation Mechanism. Adv. Mater. 33, 2100574 (2021).
Spotte-Smith, E. W. C. et al. Chemical Reaction Networks Explain Gas Evolution Mechanisms in Mg-Ion Batteries. J. Am. Chem. Soc. 145, 12181–12192 (2023).
Wen, M. et al. Chemical reaction networks and opportunities for machine learning. Nat. Comput. Sci. 3, 12–24 (2023).
Huang, H. et al. Delocalized electrolyte design enables 600 Wh kg−1 lithium metal pouch cells. Nature 644, 660–667 (2025).
Kim, M. S. et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries. Nat. Mater. 21, 445–454 (2022).
Hou, T. et al. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation. Nano Energy 64, 103881 (2019).
Islam, M. M. & van Duin, A. C. T. Reductive Decomposition Reactions of Ethylene Carbonate by Explicit Electron Transfer from Lithium: An eReaxFF Molecular Dynamics Study. J. Phys. Chem. C. 120, 27128–27134 (2016).
Fong, K. D., Self, J., McCloskey, B. D. & Persson, K. A. Onsager transport coefficients and transference numbers in polyelectrolyte solutions and polymerized ionic liquids. Macromolecules 53, 9503–9512 (2020).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Zhang, Y.-W. et al. Roadmap for the development of machine learning-based interatomic potentials. Model. Simul. Mater. Sci. Eng. 33, 023301 (2025).
Ko, T. W. & Ong, S. P. Recent advances and outstanding challenges for machine learning interatomic potentials. Nat. Comput. Sci. 3, 998–1000 (2023).
Choudhary, K. et al. Recent advances and applications of deep learning methods in materials science. npj Comput Mater. 8, 59 (2022).
Cheng, B. Cartesian atomic cluster expansion for machine learning interatomic potentials. npj Comput. Mater. 10, 157 (2024).
Keith, J. A. et al. Combining Machine Learning and Computational Chemistry for Predictive Insights Into Chemical Systems. Chem. Rev. 121, 9816–9872 (2021).
Elena, A. M. et al. Machine learned potential for high-throughput phonon calculations of metal—organic frameworks. npj Comput Mater. 11, 125 (2025).
Schütt, K. et al. Schnet: A continuous-filter convolutional neural network for modeling quantum interactions. Adv. Neural Inf. Process. Syst. 30 (2017).
Chen, C., Ye, W., Zuo, Y., Zheng, C. & Ong, S. P. Graph Networks as a Universal Machine Learning Framework for Molecules and Crystals. Chem. Mater. 31, 3564–3572 (2019).
Chen, C. & Ong, S. P. A universal graph deep learning interatomic potential for the periodic table. Nat. Comput. Sci. 2, 718–728 (2022).
Ko, T. W. & Ong, S. P. Data-efficient construction of high-fidelity graph deep learning interatomic potentials. npj Comput. Mater. 11, 65 (2025).
Deng, B. et al. CHGNet as a pretrained universal neural network potential for charge-informed atomistic modelling. Nat. Mach. Intell. 5, 1031–1041 (2023).
Ko, T. W. et al. Materials Graph Library (MatGL), an open-source graph deep learning library for materials science and chemistry. npj Comput Mater. 11, 253 (2025).
Li, X.-L. et al. Stabilizing Transition Metal Vacancy Induced Oxygen Redox by Co2+/Co3+ Redox and Sodium-Site Doping for Layered Cathode Materials. Angew. Chem. Int. Ed. 60, 22026–22034 (2021).
Zhao, C. et al. Rational design of layered oxide materials for sodium-ion batteries. Science 370, 708–711 (2020).
Wu, L.-T. et al. Prediction of Structural Stability of Layered Oxide Cathode Materials: Combination of Machine Learning and Ab Initio Thermodynamics. Adv. Energy Mater. e05470, https://doi.org/10.1002/aenm.202505470 (2025).
Köster, K., Binninger, T. & Kaghazchi, P. Optimization of Coulomb energies in gigantic configurational spaces of multi-element ionic crystals. npj Comput. Mater. 11, 202 (2025).
Liu, Y. et al. Mitigation of Jahn–Teller distortion and Na+/vacancy ordering in a distorted manganese oxide cathode material by Li substitution. Chem. Sci. 12, 1062–1067 (2021).
Li, Y. et al. Enhanced stability in a layered P2-Na0.67Fe0.5Mn0.5O2 cathode for sodium ion batteries via a synergistic Cu/Ti co-doping strategy. Inorg. Chem. Front. https://doi.org/10.1039/D5QI01070E (2025).
Jin, J. et al. Intrinsic distortion against Jahn-Teller distortion: a new paradigm for high-stability Na-ion layered Mn-rich oxide cathodes. Angew. Chem. Int. Ed. 64, e202423728 (2025).
Tian, K. et al. Boosting electrochemical reaction and suppressing phase transition with a high-entropy O3-type layered oxide for sodium-ion batteries. J. Mater. Chem. A 10, 14943–14953 (2022).
Xiong, X. et al. Atomic-level electric polarization in entropy-driven perovskites for boosting dielectric response. Adv. Mater. 37, 2415351 (2025).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Pearson, R. G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 45, 581 (1968).
Pearson, R. G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 45, 643 (1968).
Murty, B. S., Yeh, J.-W., Ranganathan, S. & Bhattacharjee, P. P. High-entropy alloys. (Elsevier, 2019).
Zhang, R.-Z. & Reece, M. J. Review of high entropy ceramics: design, synthesis, structure and properties. J. Mater. Chem. A 7, 22148–22162 (2019).
Takahashi, Y., Gotoh, Y. & Akimoto, J. Single-crystal growth, crystal and electronic structure of NaCoO2. J. Solid State Chem. 172, 22–26 (2003).
Horton, M. K. et al. Accelerated data-driven materials science with the Materials Project. Nature Materials, https://doi.org/10.1038/s41563-025-02272-0 (2025).
Jain, A. et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 1, https://doi.org/10.1063/1.4812323 (2013).
Huang, Q. et al. Coupling between electronic and structural degrees of freedom in the triangular lattice conductor NaxCoO2. Phys. Rev. B 70, 184110 (2004).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, https://doi.org/10.1063/1.3382344 (2010).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Pack, J. D. & Monkhorst, H. J. Special points for Brillouin-zone integrations”-a reply. Phys. Rev. B 16, 1748–1749 (1977).
Jain, A. et al. Formation enthalpies by mixing GGA and GGA + U calculations. Phys. Rev. B 84, 045115 (2011).
Jain, A. et al. A high-throughput infrastructure for density functional theory calculations. Comput. Mater. Sci. 50, 2295–2310 (2011).
Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984).
Hoover, W. G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Metropolis, N., Rosenbluth, A. W., Rosenbluth, M. N., Teller, A. H. & Teller, E. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 21, 1087–1092 (1953).
Acknowledgements
This work has been financially supported by the National Science and Technology Council, Taiwan (NSTC 113-2113-M-011-003-MY3, 114-2639-E-011-001-ASP, 115-2927-I-011-502, and 114-2923-E-011-001) and Sustainable Electrochemical Energy Development (SEED) Center supported by the Ministry of Education (MOE), Taiwan. The authors thank the Taiwan National Center of High-Performance Computing (NCHC) for computing resources. We also acknowledge support from the UMC Fellowship.
Author information
Authors and Affiliations
Contributions
L.T.W., Z.L.L., and S.Y.Y. designed and performed the computations under the supervision of P.K. and J.C.J. All authors discussed the computational results. L.T.W. wrote the manuscript with support from Z.L.L. and S.Y.Y. P.K., and J.C.J. contributed to revising the manuscript. J.C.J. served as the lead principal investigator.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, LT., Li, ZL., Yen, SY. et al. Probing entropic control of stacking phase preference in layered oxide cathodes for sodium-ion batteries via machine-learning potentials. npj Comput Mater (2026). https://doi.org/10.1038/s41524-025-01954-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-025-01954-2