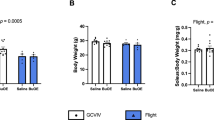
Abstract
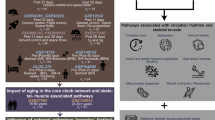
Age-related skeletal muscle deterioration, referred to as sarcopenia, poses significant risks to astronaut health and mission success during spaceflight, yet its multisystem drivers remain poorly understood. While terrestrial sarcopenia manifests gradually through aging, spaceflight induces analogous musculoskeletal decline within weeks, providing an accelerated model to study conserved atrophy mechanisms. Here, we introduced an integrative framework combining cross-species genetic analysis with physiological modeling to understand mechanistic pathways in space-induced sarcopenia. By analyzing rodent and human datasets, we identified conserved molecular pathways underlying spaceflight-induced muscle atrophy, revealing shared regulators of neuromuscular signaling including pathways related to neurotransmitter release and regulation, mitochondrial function, and synaptic integration. Building upon these molecular insights, we developed a physiologically grounded central pattern generator model that reproduced spaceflight-induced locomotion deficits in mice. This multi-scale approach established mechanistic connections between transcriptional changes and impaired movement kinetics while identifying potential therapeutic targets applicable to both spaceflight and terrestrial aging-related muscle loss.
Similar content being viewed by others
Data availability
All human transcriptomics data used from the study are available through Gene Expression Omnibus with accession numbers GSE14901, GSE111006, GSE111010, and GSE111016. Mouse transcriptomics data is available through the NASA Open Science Data Repository with accession OSD-103. Mouse behavioral data is available with accession OSD-478 on the NASA Open Science Data Repository.
Code availability
All code used for the analysis is available at https://github.com/Brubaker-Lab/MouseFLTandHumanSA. All supplementary information, including figures and data, is also made available on the GitHub repository.
References
Yatagai, F., Honma, M., Dohmae, N. & Ishioka, N. Biological effects of space environmental factors: a possible interaction between space radiation and microgravity. Life Sci. Space Res. 20, 113–123 (2019).
Chancellor, J. C., Scott, G. B. I. & Sutton, J. P. Space Radiation: The number one risk to astronaut health beyond low earth orbit. Life (Basel) 4, 491–510 (2014).
Cucinotta, F. A. Space radiation risks for astronauts on multiple international space station missions. PLoS ONE 9, e96099 (2014).
Willey, J. S. et al. The individual and combined effects of spaceflight radiation and microgravity on biologic systems and functional outcomes. J. Environ. Sci. Health, C 39, 129–179 (2021).
Le, H., Rai, V. & Agrawal, D. K. Cholesterol: an important determinant of muscle atrophy in astronauts. J. Biotechnol. Biomed. 6, 67–79 (2023).
Trappe, T. A., Tesch, P., Alkner, B. & Trappe, S. Microgravity-induced skeletal muscle atrophy in women and men: implications for long-duration spaceflights to the Moon and Mars. J. Appl. Physiol. 135, 1115–1119 (2023).
Dhillon, R. J. & Hasni, S. Pathogenesis and management of sarcopenia. Clin. Geriatr. Med. 33, 17–26 (2017).
Takahashi, H., Nakamura, A. & Shimizu, T. Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem. Biophys. Res. Commun. 578, 115–121 (2021).
Larsson, L. et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev. 99, 427–511 (2019).
Corlett, T., Stavnichuk, M. & Komarova, S. V. Population analysis of space travelers. Life Sci. Space Res. 27, 1–5 (2020).
Kunitskaya, A., Piret, J. M., Buckley, N. & Low-Décarie, E. Meta-analysis of health research data from greater than three months International Space Station missions. Acta Astronautica 201, 420–430 (2022).
Kim, S., Ayan, B., Shayan, M., Rando, T. A. & Huang, N. F. Skeletal muscle-on-a-chip in microgravity as a platform for regeneration modeling and drug screening. Stem Cell Rep. 19, 1061–1073 (2024).
Baek, K.-W. et al. Rodent model of muscular atrophy for Sarcopenia study. J. Bone Metab. 27, 97–110 (2020).
Brubaker, D. et al. An interspecies translation model implicates integrin signaling in infliximab-resistant inflammatory bowel disease. Sci. Signal 13, eaay3258 (2020).
Brubaker, D. K. & Lauffenburger, D. A. Translating preclinical models to humans. Science 367, 742–743 (2020).
Bergendorf, A., Park, J. H., Ball, B. K. & Brubaker, D. K. Mouse-to-human modeling of microglia single-nuclei transcriptomics identifies immune signaling pathways and potential therapeutic candidates associated with Alzheimer’s disease. 2025.02.07.637100 https://doi.org/10.1101/2025.02.07.637100 (2025).
Ball, B. K., Proctor, E. A. & Brubaker, D. K. in Biocomputing 2025 426–440 (World Scientific, 2024). https://doi.org/10.1142/9789819807024_0031.
Lee, M. J. et al. Computational interspecies translation between Alzheimer’s disease mouse models and human subjects identifies innate immune complement, TYROBP, and TAM receptor agonist signatures, distinct from influences of aging. Front. Neurosci. 15 (2021).
Ball, B. K., Park, J. H., Bergendorf, A. M., Proctor, E. A. & Brubaker, D. K. Translational disease modeling of peripheral blood identifies type 2 diabetes biomarkers predictive of Alzheimer’s disease. npj Syst. Biol. Appl 11, 1–16 (2025).
Frost, M. R. et al. Computational translation of mouse models of osteoarthritis predicts human disease. Osteoarthritis Cartilage 0 (2025).
Suarez-Lopez, L. et al. Cross-species transcriptomic signatures predict response to MK2 inhibition in mouse models of chronic inflammation. iScience 24, 103406 (2021).
Harris-Warrick, R. M. Neuromodulation and flexibility in Central Pattern Generator networks. Curr. Opin. Neurobiol. 21, 685–692 (2011).
Grillner, S. & El Manira, A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol. Rev. 100, 271–320 (2020).
Camera, A. et al. Aging and putative frailty biomarkers are altered by spaceflight. Sci. Rep. 14, 13098 (2024).
Parafati, M., Thwin, Z., Malany, L. K., Coen, P. M. & Malany, S. Microgravity accelerates skeletal muscle degeneration: functional and transcriptomic insights from an ISS muscle lab-on-chip model. Stem Cell Rep. 20 (2025).
Cannavo, A. et al. Are skeletal muscle changes during prolonged space flights similar to those experienced by frail and sarcopenic older adults?. Life 12, 2139 (2022).
Okada, R. et al. Transcriptome analysis of gravitational effects on mouse skeletal muscles under microgravity and artificial 1 g onboard environment. Sci. Rep. 11, 9168 (2021).
Hayashi, T. et al. Lunar gravity prevents skeletal muscle atrophy but not myofiber type shift in mice. Commun. Biol. 6, 424 (2023).
Murgia, M. et al. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep. 19, 2396–2409 (2017).
Beheshti, A., Ray, S., Fogle, H., Berrios, D. & Costes, S. V. A microRNA signature and TGF-β1 response were identified as the key master regulators for spaceflight response. PLoS ONE 13, e0199621 (2018).
McDonald, J. T. et al. NASA GeneLab platform utilized for biological response to space radiation in animal models. Cancers (Basel) 12, 381 (2020).
Migliavacca, E. et al. Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 10, 5808 (2019).
Membrez, M. et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat. Metab. 6, 433–447 (2024).
Chen, Y.-W. et al. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol. Genomics 31, 510–520 (2007).
Kilroe, S. P., Fulford, J., Jackman, S. R., Van Loon, L. J. C. & Wall, B. T. Temporal muscle-specific disuse atrophy during one week of leg immobilization. Med. Sci. Sports Exerc. 52, 944 (2020).
Hardy, E. J. O. et al. The time course of disuse muscle atrophy of the lower limb in health and disease. J. Cachexia, Sarcopenia Muscle 13, 2616–2629 (2022).
Flavell, S. W. & Greenberg, M. E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev. Neurosci. 31, 563–590 (2008).
Zhang, S.-J. et al. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 5, e1000604 (2009).
Hartshorne, D. J. Myosin phosphatase: subunits and interactions. Acta Physiol. Scand. 164, 483–493 (1998).
Ryder, J. W., Lau, K. S., Kamm, K. E. & Stull, J. T. Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase*♦. J. Biol. Chem. 282, 20447–20454 (2007).
Lim, S. C. et al. Mutations in LYRM4, encoding iron–sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum. Mol. Genet. 22, 4460–4473 (2013).
Mori, F. et al. Genetic variants of the NMDA receptor influence cortical excitability and plasticity in humans. J. Neurophysiol. 106, 1637–1643 (2011).
Akashi, K. et al. NMDA receptor GluN2B (GluRε2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J. Neurosci. 29, 10869–10882 (2009).
Xie, Z. et al. Getting started with LINCS datasets and tools. Curr. Protoc. 2, e487 (2022).
Ahmadian, M. et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Kwok, A. et al. Altered rodent gait characteristics after ~35 days in orbit aboard the International Space Station. Life Sci. Space Res. 24, 9–17 (2020).
Sundaramurthy, A. et al. Effect of stride length on the running biomechanics of healthy women of different statures. BMC Musculoskelet. Disord. 24, 604 (2023).
MULAVARA, A. P. et al. Physiological and functional alterations after spaceflight and bed rest. Med Sci. Sports Exerc 50, 1961–1980 (2018).
Fitts, R. H., Riley, D. R. & Widrick, J. J. Functional and structural adaptations of skeletal muscle to microgravity. J. Exp. Biol. 204, 3201–3208 (2001).
Marder, E. & Bucher, D. Central pattern generators and the control of rhythmic movements. Curr. Biol. 11, R986–R996 (2001).
Dayan, P. & Abbott, L. F. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems (MIT Press, 2001).
Trappenberg, T. Fundamentals of Computational Neuroscience (OUP Oxford, 2010).
Arnold, W. D. & Clark, B. C. Neuromuscular junction transmission failure in aging and sarcopenia: The nexus of the neurological and muscular systems. Ageing Res. Rev. 89, 101966 (2023).
Pedersen, T. H., Macdonald, W. A., Broch-Lips, M., Halldorsdottir, O. & Bækgaard Nielsen, O. Chloride channel inhibition improves neuromuscular function under conditions mimicking neuromuscular disorders. Acta Physiologica 233, e13690 (2021).
Rodríguez Cruz, P. M., Cossins, J., Beeson, D. & Vincent, A. The neuromuscular junction in health and disease: molecular mechanisms governing synaptic formation and homeostasis. Front. Mol. Neurosci. 13 (2020).
Smith, J. K. IL-6 and the dysregulation of immune, bone, muscle, and metabolic homeostasis during spaceflight. npj Microgravity 4, 1–8 (2018).
Okamura, Y. et al. Impact of microgravity and lunar gravity on murine skeletal and immune systems during space travel. Sci. Rep. 14, 28774 (2024).
An, R. et al. Influence of the spaceflight environment on macrophage lineages. npj Microgravity 10, 1–8 (2024).
Rooney, B. V., Crucian, B. E., Pierson, D. L., Laudenslager, M. L. & Mehta, S. K. Herpes virus reactivation in astronauts during spaceflight and its application on Earth. Front. Microbiol. 10, 16 (2019).
Capri, M. et al. Recovery from 6-month spaceflight at the International Space Station: muscle-related stress into a proinflammatory setting. FASEB J. 33, 5168–5180 (2019).
Crucian, B. et al. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 33, 456–465 (2013).
Wang, J. et al. The association between inflammatory cytokines and sarcopenia-related traits: a bi-directional Mendelian randomization study. Eur. J. Clin. Nutr. 78, 1032–1040 (2024).
Larouche, J. A. et al. Neutrophil and natural killer cell imbalances prevent muscle stem cell–mediated regeneration following murine volumetric muscle loss. Proc. Natl. Acad. Sci. 119, e2111445119 (2022).
Ferrando, A. A., Paddon-Jones, D. & Wolfe, R. R. Alterations in protein metabolism during space flight and inactivity. Nutrition 18, 837–841 (2002).
Stein, T. P., Leskiw, M. J., Schluter, M. D., Donaldson, M. R. & Larina, I. Protein kinetics during and after long-duration spaceflight on MIR. Am. J. Physiol.-Endocrinol. Metab. 276, E1014–E1021 (1999).
Moreira-Pais, A., Ferreira, R., Oliveira, P. A. & Duarte, J. A. A neuromuscular perspective of sarcopenia pathogenesis: deciphering the signaling pathways involved. GeroScience 44, 1199–1213 (2022).
Deschenes, M. R., Roby, M. A., Eason, M. K. & Harris, M. B. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp. Gerontol. 45, 389–393 (2010).
Beaton, L. J., Tarnopolsky, M. A. & Phillips, S. M. Contraction-induced muscle damage in humans following calcium channel blocker administration. J. Physiol. 544, 849–859 (2002).
Wagatsuma, A., Fujimoto, K. & Yamada, S. Effect of treatment with nifedipine, an L-type calcium channel blocker, on muscular atrophy induced by hindlimb immobilization. Scand. J. Med. Sci. Sports 12, 26–30 (2002).
Frick, C. G., Helming, M., Martyn, J. A. J., Blobner, M. & Fink, H. Continuous administration of pyridostigmine improves immobilization-induced neuromuscular weakness. Crit. Care Med. 38, 922 (2010).
Furukawa, S. et al. Findings from recent studies by the Japan Aerospace Exploration Agency examining musculoskeletal atrophy in space and on Earth. npj Microgravity 7, 1–10 (2021).
Lee, P. H. U., Chung, M., Ren, Z., Mair, D. B. & Kim, D.-H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Physiol.-Cell Physiol. 322, C567–C580 (2022).
Rowan, S. L., Purves-Smith, F. M., Solbak, N. M. & Hepple, R. T. Accumulation of severely atrophic myofibers marks the acceleration of sarcopenia in slow and fast twitch muscles. Exp. Gerontol. 46, 660–669 (2011).
Endo, Y. et al. Exercise-induced gene expression changes in skeletal muscle of old mice. Genomics 113, 2965–2976 (2021).
Rosa-Caldwell, M. E. et al. Sex differences in muscle health in simulated micro- and partial-gravity environments in rats. Sports Med. Health Sci. 5, 319–328 (2023).
Ploutz-Snyder, L. et al. Effects of sex and gender on adaptation to space: musculoskeletal health. J. Women’s. Health (Larchmt.) 23, 963–966 (2014).
Bloemberg, D. & Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 7, e35273 (2012).
Zhang, M. Y., Zhang, W. J. & Medler, S. The continuum of hybrid IIX/IIB fibers in normal mouse muscles: MHC isoform proportions and spatial distribution within single fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1582–R1591 (2010).
Rome, L. C. et al. Why animals have different muscle fibre types. Nature 335, 824–827 (1988).
Dolgalev, I. msigdbr: MSigDB Gene Sets for Multiple Organisms in a Tidy Data Format. https://github.com/igordot/msigdbr (2022).
Korotkevich, G. et al. Fast gene set enrichment analysis. 060012. https://doi.org/10.1101/060012 (2021).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: A J. Integr. Biol. 16, 284–287 (2012).
Rohart, F., Gautier, B., Singh, A. & Cao, K.-A. L. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 13, e1005752 (2017).
Feeney, D. F., Meyer, F. G., Noone, N. & Enoka, R. M. A latent low-dimensional common input drives a pool of motor neurons: a probabilistic latent state-space model. J. Neurophysiol. 118, 2238–2250 (2017).
Crone, S. A. & Sharma, K. Patterning spinal motor activity in the absence of synaptic excitation. Neuron 71, 957–959 (2011).
Abdelghani, M. N., Abbas, J. J., Horch, K. W. & Jung, R. A functional model and simulation of spinal motor pools and intrafascicular recordings of motoneuron activity in peripheral nerve. Front. Neurosci. 8 (2014).
Acknowledgements
B.K.B. and H.F.K. are supported by the NSF GRFP (DGE-1842166). D.D.C. is supported in part by the DARPA Young Faculty Award (Army Research Office Contract W911NF21103272) and National Science Foundation (Awards 1944394 and 2149946). D.K.B. is supported by an award from the Good Ventures Foundation, Open Philanthropy, and start-up funds from Case Western Reserve University.
Author information
Authors and Affiliations
Contributions
B.K.B.: Conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft, writing-review & editing. H.F.K.: Conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing-original draft, writing-review & editing. J.H.P.: Data curation, methodology, writing-review & editing. K.J.: Project administration, resources, writing-review & editing. D.D.C.: Conceptualization, methodology, project administration, resources, funding acquisition, writing-review & editing. D.K.B.: Conceptualization, funding acquisition, methodology, project administration, resources, writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ball, B.K., Khan, H.F., Park, J.H. et al. Integrated cross-species translation and biophysical multi-scale modeling links molecular signatures and locomotory phenotypes in spaceflight-induced sarcopenia. npj Microgravity (2026). https://doi.org/10.1038/s41526-025-00557-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-025-00557-x