Abstract
The pitting corrosion resistance and mechanical behavior of nickel-free high nitrogen stainless steel (HNS) and its fabricated stents under varying cold deformation conditions were systematically evaluated, benchmarked against clinically deployed stent materials. Electrochemical analyses demonstrate that while aggressive cold deformation reduces the pitting resistance of HNS in physiological saline, this degradation is fully counteracted when nitrogen content reaches 0.92 wt.%. This abnormal behavior of HNS is owed to the N enrichment as SRO [CrN] in the inner layer of the passive film. Post-deformation evaluations confirm that HNS stents maintain superior pitting resistance compared to L605 and 316 L stents following compressive and over-expansion loading. Immersion tests coupled with cellular assays reveal that manganese ion release from HNS had protective effect on the activity of cellular manganese superoxide dismutase (MnSOD). The optimized strength-ductility synergy observed in cold- deformed HNS correlates with preliminary in vivo stent integrity assessments. These findings collectively suggest that HNS stents may offer enhanced clinical safety profiles through combined corrosion resistance and biomechanical compatibility advantages.
Similar content being viewed by others
Introduction
Nowadays, coronary stents have been widely used in the treatment of coronary artery stenosis. However, accumulating clinical evidence over the past three decades has revealed stent fracture as a critical complication, with reported incidence rates exceeding 20% in certain patient cohorts1,2,3. This mechanical failure mode can precipitate severe clinical sequelae, including vascular perforation, in-stent restenosis, and thrombosis, posing significant risks to patient safety. From a materials science perspective, these challenges underscore the urgent need for advanced stent alloys with optimized corrosion resistance and mechanical durability to mitigate fracture-related complications.
Current clinical applications of coronary stents primarily utilize L605 cobalt-chromium alloy and 316 L stainless steel. Nickel-free high nitrogen stainless steel (HNS) has emerged as a promising next-generation stent material due to its superior combination of mechanical and corrosion-resistant properties4,5,6. During fabrication and service, coronary stents undergo substantial cold deformation through compression and expansion processes. This manufacturing-induced strain significantly compromises the pitting corrosion resistance of conventional stainless steels7,8,9, consequently elevating fracture risks in clinical deployment. These operational constraints underscore the critical importance of maintaining material performance under cold-worked conditions.
Experimental evidence confirms that nitrogen alloying effectively enhances pitting corrosion resistance in stainless steel systems through metastable pit stabilization mechanisms10. Additionally, cold-working processes typically enhance the strength of metallic alloys but simultaneously reduce ductility. Recent studies demonstrate that nitrogen alloying not only improves tensile strength but also maintains excellent ductility for stainless steel11,12. On this basis, as a stent material, HNS with high nitrogen content is expected to achieve improved balance of strength and ductility, and keep an excellent pitting corrosion resistance under cold-worked state.
In general, in order to increase the solubility of nitrogen, manganese was added to HNS. There were differing opinions on biosafety studies of manganese. Some scholars believed that manganese overexposure was associated with dopaminergic dysfunction13 and neurological abnormalities14, and that the accumulation of manganese in tissues may cause manganese poisoning15,16,17. In addition, some studies have shown that manganese has low toxicity, and manganese derivatives are widely used in tumor treatment and biomedical materials18,19. As an essential trace element, manganese ions (Mn²⁺) serve as cofactors for multiple enzymes involved in carbohydrate metabolism, antioxidant defense, and neural signaling20,21. While demonstrating significant advantages in immunotherapy through immunomodulatory effects, excessive Mn²⁺ accumulation (>300 μM) may induce neurotoxicity and metabolic disturbances, necessitating precise dosage control to balance therapeutic efficacy with biosafety22. Although our previous studies have shown that the Fe-30Mn-0.6 N alloy has good biosafety in vivo23, the protective effect of trace Mn²⁺ dissolution from HNS on cells has been infrequently reported. Mn²⁺ is a crucial component of manganese superoxide dismutase (MnSOD)24, which is mainly responsible for scavenging reactive oxygen species (ROS) generated by mitochondrial oxidative stress. MnSOD has the functions of regulating smooth muscle cells, promoting vascular repair25 and maintaining the reduced state of iron26.
In this study, nickel-free HNS with two nitrogen levels was prepared. The effects of cold deformation on the performance of HNS and stents were systematically investigated and compared with L605 cobalt-based alloy and 316 L stainless steel. The effects of HNS on MnSOD activity in human umbilical vein endothelial cells (HUVEC) and the fractures of stents after in vivo implantation were preliminarily investigated.
Results and discussion
Corrosion behavior
Figure 1a, b show the potentiodynamic curves of 0.76 N and 0.92 N HNS at different cold deformation levels in 0.9 wt.% NaCl solution at 37°C. Given Cl ions play an important role in the pitting corrosion of stainless steel, 0.9 wt.% NaCl was selected as the electrochemical test solution rather than complex simulated body fluids. When the anodic current density monotonically increased to 100 μA/cm2, the potential was defined as the pitting potential (Ec) according to ISO 15,158:2014. Initially, for 0.76 N HNS, the shapes of potentiodynamic curves were not influenced by cold deformation. But, after severe cold deformation (beyond 20% reduction in thickness), the passive regions became unstable, and the Ec of cold-deformed sample drops dramatically. Many researchers believed that the deformation band produced in deformation process should be responsible for such detrimental effect7,9,27. However, for 0.92 N HNS, the potentiodynamic curves and Ec were not influenced by severe cold deformation. And the detrimental effect was almost completely eliminated. This is consistent with the HNS in the simulated seawater, as reported before14. According to this, if the nitrogen content is high enough, the HNS should acquire an excellent pitting corrosion resistance even after severe deformation. This special effect of nitrogen has been fully discussed in our previous studies, which was attributed to the nitrogen enrichment in the passive film on HNS28,29.
To further investigate the passive film of HNS in saline, 0.76 N and 0.92 N were polarized at a potential in passive region. Figure 2a, b show the Bode plots of passive film polarized at 0.3 V for 1 h for HNS at different cold deformation levels.To make sure the passive film was stable, the polarization time was selected as 1 h. In fact, the variation tendency of passive film of HNS polarized at 0.1 V and 0.2 V (provided in Supplementary Figs. 1, 2), is the same as 0.3 V. Hence, only EIS tests results at 0.3 V are shown.
From the phase curves, two time constants were seen for all specimens, which represents the capacitance of the passive film and the double layer. From the impedance curves, there was no obvious difference in the low frequency range for two HNS at different cold deformation levels. However, in the high frequency range, it seems that the impedance of 0.76 N slightly decreases and that of 0.92 N obviously increases after cold deformation. Nyquist plots in Fig. 2c and Fig. 2d indicate that, after cold deformation, the curvature radius of the capacitive loop obviously decreases for 0.76 N and increases for 0.92 N. Generally, a higher curvature radius of capacitive reactance arc represents stronger corrosion resistance.
To further analyze the EIS results, an equivalent circuit shown in Fig.3a was used to fit the electrochemical impedance spectroscopy (EIS) data in Fig.2. In this model, Rsol represents the solution resistance, R1 and R2 are the passive film resistance and the charge transfer resistance, respectively. C and Q represent the capacitance of the passive film and the double layer, respectively. Figure 3b shows that, for 0.76 N, as deformation levels increased, the passive film resistance significantly decreased, but for 0.92 N, the passive film resistance obviously increased. Figure 3c shows that, as deformation levels increased, the passive film capacitance of 0.76 N slightly decreased at first and then obviously increased, but that of 0.92 N obviously decrease.
As we all know, the capacitance could be calculated by the following formula: C = εS/d. When the passive film was believed to be pure capacitor, ε represents the dielectric constant of passive film, S and d represent the area and thickness of passive film. If we assume that the ε value is constant for passive film, the capacitance of passive film was inversely proportional to its thickness. Thus, we could conclude that, after severe deformation, the resistance and thickness of passive film obviously decreased and increased for 0.76 N and 0.92 N, respectively. The behaviors of the passive film corresponded to the pitting corrosion resistance obtained from potentiodynamic tests.
By secondary ion mass spectroscopy (SIMS) analyses, the elements spatial distributions of passive film for 0.76 N were shown in Fig.4. Obviously, Fig.4a indicates that N detected as CrN- signal seriously enriched in the passive film for HNS. This is totally different with passive film for traditional stainless steel without N, which only consist of Fe and Cr enrichment layers. Worth noting that the spatial distribution of N for HNS passive film was firstly presented, and we could visually see it.
From Fig.4b, SIMS results show that N enriched in the inner layer of Cr enriched passive film as short range ordered [CrN] clusters. This special [CrN] enrichment structure of passive film on HNS is totally different with traditional stainless steel without nitrogen. It was easy to conclude that, as severe cold deformation occurred, the outer layer passive film could be broken. Then, as we know, [CrN] was not stable, which could react with solution and generate Cr2O3 as new passive film. The reaction was shown as follows: [CrN]+ H2O → Cr2O3 + NH3. This reaction only happens in HNS with high enough N and [CrN] enrichment in passive film. Thus, it was easy to understand the abnormal pitting corrosion resistance for 0.92 N even after severe cold deformation.
As we all know, the human blood is very complicated. There are many ions, protein, cells, etc., which also have effect on the ion dissolution. In this study, to simplify the test and keep high ion concentration measurement accuracy, the 0.9 wt.% NaCl solution was selected as immersion solution. Table 1 shows that, due to the chemical instabilities of passive film, Fe ions are the element with the largest dissolution amount for HNS, Fe ions are that for 316 L stainless steel, and Co ions are that for L605 cobalt-based alloy.
For HNS, as Fe is the base element, it is easy to understand that the amount of Fe ions dissolved is the highest. Although there is only about 15 wt.% Mn in HNS, the Mn ions dissolution amount is almost half of Fe ions, which attracted our attentions. This behavior is the same for two HNS. According to the Lange’s hand book of chemistry (Sixteenth Edition), the solubility product of Fe and Mn ions precipitates Fe(OH)3 and Mn(OH)2 are 2.79 × 10-39 and 4.0 × 10-14, respectively. It means that Fe ions are easier to produce precipitates than Mn ions, which would prevent the further dissolution of ions. This should be the reason for the high dissolution rate of Mn ions with low content.
Meanwhile, from the perspective of biological safety, the Mn adaptation in HUVECs has been preliminarily verified in subsequent cell experiments. We have studied the effects of Mn ions dissolution from HNS on MnSOD activity in HUVECs, and it will be discussed below. Studies have shown that excessive amounts of Co ions can cause hazards such as cardiomyopathy in humans30. In addition, both 316 L stainless steel and L605 alloy will dissolve a certain amount of Ni ions. A large number of studies have shown that the dissolution of small amounts of Ni ions may bring serious allergenic and teratogenic hazards to the human body4,30,31.
Mechanical properties
Tensile strength and elongation of 0.76 N, 0.92 N, L605 and 316 L at different cold working levels are compared. Figure 5a shows that under the solution treated state, as nitrogen content increased from 0.76 wt.% to 0.92 wt.%, both ultimate strength and yield strength of HNS were enhanced. Besides, the yield strengths of both solution-treated 0.76 N and 0.92 N were higher than that of L605, but the ultimate strength of 0.76 N was slightly lower than that of L605. Compared with 316 L, the strengths of two HNSs increased almost for twice. After cold deformation, all the materials behave strong work hardening behaviors. Among them, the work hardening ability of L605 was the strongest, and that of two HNSs was slightly weaker than L605. 316 L behaved the weakest work hardening ability, and its strength was much lower than the other materials.
Figure 5b shows that, under the solution treated state, all the materials behaved excellent plasticity. Among them, the elongation of 316 L was slightly higher than the others. For the two HNSs and L605, their plasticity was almost the same. After 20% cold deformation, the plasticity reduction of L605 was the highest, and that of 316 L took the second place. However, the two HNSs still kept about 20% elongation. After severe cold deformation, L605 behaved brittle, but HNS and 316 L still had 6 ~ 10% elongation.
Based on the above results, the cold-deformed HNS and L605 had similar strength that was much higher than 316 L. Meanwhile, the cold-deformed HNS and 316 L had similar plasticity that was much higher than L605. Thus, HNS could achieve a better match of strength and plasticity. This behavior of HNS should be attributed to the effect of nitrogen alloying11,32. Besides, because of the combination of good pitting corrosion resistance and mechanical properties, our previous study found that HNS could achieve an excellent corrosion fatigue resistance33. Based on the above results, HNS with 0.94 wt.% nitrogen was used to manufacture coronary stents.
Stents cold-deformed morphologies and corrosion behavior
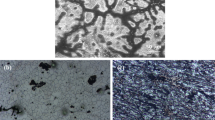
The cold deformation processes of compression, expansion, and over-expansion of the stents before service were briefly described, as illustrated in Supplementary Fig. 3. Figure 6a–c shows the surface morphologies of the largest deformation area on HNS stents. It can be seen that, before deformation, the surface of HNS stent was very smooth. After compressing and expanding to 4.0 mm in diameter, slip bands appeared and the surface quality was still good. However, after over expanding to 5.5 mm in diameter, the number of slip bands significantly increased, presenting cross slip bands. Additionally, because of inhomogeneous deformation, the surface irregularity of stents obviously increased.
a SEM morphology of HNS stent with no deformation; (b) SEM morphology of HNS stent after compressing and expanding to diameter of 4.0 mm; (c) SEM morphology of HNS stent after expanding to diameter of 5.5 mm; (d) Potentiodynamic curve of HNS stent at different deformation levels; (e) Potentiodynamic curve of L605 stent at different deformation levels; (f) Potentiodynamic curve of passivated 316 L stent at different deformation levels.
Figure 6d–f presents the potentiodynamic curves of HNS stent, L605 stent and passivated 316 L stent at different deformation levels. As expected, even after compressing and over expanding, the potentiodynamic curves of HNS stent were almost not influenced. It is noteworthy that HNS stents exhibited the highest Ec, which was not influenced by compression or over expansion. Initially, the potentiodynamic curve of passivated 316 L stent was also not influenced. However, after over expansion, the passivation region became unstable, and Ec of passivated 316 L stent reduced to half, which is close to that of 316 L without passivation. That means the rupture of passive film happened. Until now, 316 L stents are still widely used in clinic. However, this risk of the deformed 316 L stents has never been paid attention. Although the L605 stent remained unaffected, its Ec registered the lowest value except for the over-expanded 316 L. The formation of surface pits could serve as initiation sites for stent fractures. In this context, the HNS stent demonstrated superior fracture resistance due to its elevated nitrogen content, suggesting enhanced clinical safety compared to currently utilized stent materials.
Actually, owing to the existence of pulsatile blood flow, the service conditions of stents in diseased coronary arteries are very complex. It is very difficult to simulate the real condition of stents or directly characterize their corrosion resistance. In this case, we used the pitting corrosion potential, to evaluate the effect of cold deformation on the pitting corrosion resistance of stents.
MnSOD activity of cells
The immersion experiment (Table 1) indicates that the concentration of Mn ions leached from HNS in the short term is higher than that from both L605 and 316 L materials, making it necessary to study MnSOD activity in cells. To evaluate the effect of the materials on MnSOD activity in endothelial cells, HUVECs were cultured on Control, 0.92 N, L605, and 316 L groups for 3 days, respectively. Compared with the other three groups, the MnSOD activity was significantly decreased of cells exposed to L605 (P < 0.001) (Fig. 7). This may be attributed to the toxicity associated with a large amount of Co ions leaching from L60534. The MnSOD activity of cells cultured with 316 L was significantly reduced compared to the control group (P < 0.01), and with no significant difference observed in the 0.92 N group (P = 0.13). In comparison to the control group, the 0.92 N group exhibited minimal changes in MnSOD activity. It is hypothesized that enhanced antioxidant capacity over longer durations would positively influence subsequent cell viability and proliferation.
In vivo fractograph of stents implantation
After the in vivo experiments, the coronary arteries were collected in the dead pigs caused by artery break and thrombosis. Through HRTXRT analyses, it was found that one L605 stent fractured and one 316 L stent fractured in the arteries, respectively. Figure 8 shows mesh structures of these fractured stents. Figure 8a illustrates that the L605 stent was compressed and flattened along the radial direction, and the fracture happened at two positions of the circular strut. Figure 8c illustrates that the 316 L stent was drawn and compressed along the axial direction, and the fracture happened at four positions of the links.
Actually, owing to the existences of hard calcification plaques and cyclic radial force, the service condition of stents in the diseased coronary arteries is very complex. It is very difficult to simulate the real condition of stents or directly characterize their mechanical properties. However, as shown in Fig. 8, the fractographs of L605 and 316 L stents indicate that their ductility and strength were not high enough, respectively. This result is consistent with the mechanical properties of stent materials after deformation, as shown in Fig. 2. Although the fracture incidence (0/47 for HNS vs. 1/24 for L605 vs. 1/39 for 316 L) did not reach statistical significance (P > 0.05), the data suggest a favorable trend for HNS stents in resisting mechanical failure. Besides, owing to avoiding the allergy reactions caused by Ni ions released from stents, HNS stents did reduce the occurrence of in-stent restenosis4,35. More importantly, HNS stents will solve the problem patients with seriously Ni allergy cannot implant stents.
This study constitutes the first systematic comparison of the influences of deformation on the stent materials and stents. The cold-deformed HNS and corresponding stents exhibited enhanced pitting corrosion resistance and superior mechanical integrity compared to conventional counterparts. These findings hold significant clinical implications for reducing fracture-related failure rates and improving prognostic outcomes. Notably, preliminary validation confirmed that manganese ions released from HNS did not induce significant alterations in MnSOD expression within HUVECs. However, given the multifactorial nature of stent fracture, future research should prioritize comprehensive risk stratification through mechanistic investigations, while advancing innovative engineering approaches to mitigate deformation-induced failure modes.
In the physiological saline, severe cold deformation seriously weakened the pitting corrosion resistance of HNS, but when the nitrogen content was high enough, this detrimental effect could be completely eliminated. This abnormal behavior of HNS is owed to the N enrichment as SRO [CrN] in the inner layer of passive film. As expected, even after compressing and over expanding, HNS stents maintained much better pitting corrosion resistance than L605 and 316 L stents. Besides, under the cold-deformed state, HNS could achieve better match of strength and plasticity, which is consistent with the preliminary in vivo analysis of sent fracture. Hence, the HNS stents should achieve a higher safety than the clinically used stents.
Methods
Sample preparation
Nickel-free HNSs (Fe-18Cr-15Mn-2.5Mo-N) with 0.76 wt.% and 0.92 wt.% nitrogen contents, respectively, were studied, which are named as 0.76 N and 0.92 N in the context. The details of their chemical compositions have been mentioned before28. The 316 L stainless steel and L605 cobalt-based alloy are commercial stent materials. The as-received HNS plates (10 mm thick) and 316 L plates (5 mm thick) were solution treated at 1150 °C for 1 h, and L605 plates (5 mm thick) were solution treated at 1200 °C for 1 h. Then all the plates were quenched in water. By multi-pass unidirectional cold rolling, the solution treated plates were respectively cold deformed to 10, 20, 30, 40, 50 and over 60% reductions in thickness.
Coronary stents made of HNS with 0.92 wt.% nitrogen, L605 and 316 L were manufactured. All the coronary stents were subjected to laser cutting, acid pickling and electrochemical polishing. To obtain high pitting corrosion resistance, nitric acid passivation was conducted for 316 L stents. The strut thickness of HNS, L605 and 316 L stents were 80 μm, 100 μm and 120 μm, respectively.
Corrosion test
Potentiodynamic tests were performed to investigate the pitting corrosion resistance of materials and stents in naturally aerated 0.9 wt.% NaCl solution at 37 °C. The process of samples preparation was the same with our previous study28. In the tests, a Gamry Reference 600 was applied, and the scan rate was of 0.1667 mV/s. From the potentiodynamic tests, the cold working effects on the pitting corrosion resistance of HNS in normal saline were almost the same as in the simulated sea water28. To further investigate their passive films and facilitate comparison with numerous previous studies obtained in simulated sea water, after being polarized at 0.3 V for 1 h, EIS tests in simulated sea water NaCl solution were performed at 0.3 V. The frequency range was from 100 kHz to 10 mHz. The excitation signal was a sinusoidal voltage with an amplitude of 10 mV. Gamry Echem Analyst software was used to analyze the data. Besides, the composition of passive film obtained by polarization at 0.3 V for 1 h was analyzed by a Tof-SIMS 5 spectrometer (IonTof), and the details were following the same procedure as our previous research28.
The 0.76 N, 0.92 N HNS samples, L605 alloy and 316 L stainless steel samples were immersed in 0.9 wt.% NaCl solution at 37 °C for 7 days. Immersion ratio was 0.8 ml/cm2 according to ISO 10993-15:2000. The ion concentration in the immersion solution was detected by atomic absorption spectroscopy (AAS) Z000, in which Fe, Mn and Co were determined by flame method, and Ni, Cr and Mo were determined by graphite furnace method. In addition, the W concentration in the immersion solution was determined by inductively coupled plasma-mass spectrometry (ICP-MS) iCAP-Qc.
Mechanical property test
Dog-bone-shaped tensile samples with the gauge dimensions of 20 mm×4 mm×1 mm were cut perpendicular to the rolling direction. All the specimens were ground to 1000 grit finish before test. Tensile tests were performed on an AG-100kNG mechanical testing machine, and the setting experimental conditions obeyed the ISO 6892-1. The yield strength, ultimate strength and elongation of specimens were obtained.
Structural characterization of cold deformation of stents
The initial outer diameter of HNS, L605 and 316 L stents was 1.8 mm. All stents were compressed to 1.0 mm on balloon catheters, then expanded to 4.0 mm and over expanded to 5.5 mm by balloons. Before and after deformation, the surface morphology of stents was observed using a FEI Nano450 scanning electron microscope (SEM).
MnSOD activity assay
HUVECs were purchased from Procell Life Science&Technology Co.,Ltd. Cells were cultured in EGM-2 medium and 5% CO2, in a 37°C humidified atmosphere. HUVECs were cultured on metal-free samples (Control group), HNS (0.92 N group), L605, and 316 L samples for 3 days, respectively. Total SOD activity was assessed using the xanthine-xanthine oxidase and nitro blue tetrazolium (NBT) diformazan method. KCN was used to inhibit Cu/ZnSOD activity. Absorbance was measured at 560 nm to measure NBT reduction. 50% inhibition of NBT reduction equals to 1 unit of SOD activity. Each experimental group was established with three replicate wells, and the experiment was independently repeated three times. Statistical analysis and graphing were performed using GraphPad Prsim9, and the final results were expressed as mean ± standard deviation (SD). When the data interpretation was P < 0.05, the difference was statistically significant.
In Vivo analysis
To investigate the fracture behaviors of coronary stents in service, the fractured stents were collected from our previous in vivo experiments, in which 47 HNS stents, 24 L605 stents and 39 316 L stents were implanted in porcine coronary arteries. Part of histopathologic data have been reported36. High resolution transmission X-ray tomography (HRTXRT), Xradia Versa XRM-500, was applied to analyze the structural integrity of fractured stents in arteries37.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Nakazawa, G. et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J. Am. Coll. Cardiol. 54, 1924–1931 (2009).
Acar, R. D., Bulut, M. & Akcakoyun, M. Are we aware of stent fracture?. Herz 40, 417–422 (2015).
Kan, J. et al. Incidence and clinical outcomes of stent fractures on the basis of 6,555 patients and 16,482 drug-eluting stents from 4 centers. JACC Cardiovasc. Interv. 9, 1115–1123 (2016).
Fujiu, K. et al. Nickel-free stainless steel avoids neointima formation following coronary stent implantation. Sci. Technol. Adv. Mater. 13, 064218 (2012).
Talha, M., Behera, C. K. & Sinha, O. P. A review on nickel-free nitrogen containing austenitic stainless steels for biomedical applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 33, 3563–3575 (2013).
Wang, Q., Zhang, B., Ren, Y. & Yang, K. Research and application of biomedical nickel-free stainless steels. Acta Met. Sin. 53, 1311–1316 (2017).
Kamachi Mudali, U. et al. On the pitting corrosion resistance of nitrogen alloyed cold worked austenitic stainless steels. Corros. Sci. 44, 2183–2198 (2002).
Li, J. et al. Enhancing pitting corrosion resistance of severely cold-worked high nitrogen austenitic stainless steel by nitric acid passivation. J. Electrochem. Soc. 166, C365–C374 (2019).
Fu, Y. et al. Influence of cold work on pitting corrosion behavior of a high nitrogen stainless steel. J. Electrochem. Soc. 155, C455 (2008).
Ningshen, S., Kamachi Mudali, U., Mittal, V. K. & Khatak, H. S. Semiconducting and passive film properties of nitrogen-containing type 316LN stainless steels. Corros. Sci. 49, 481–496 (2007).
Simmons, J. W. Strain hardening and plastic flow properties of nitrogen-alloyed Fe-17Cr-(8–10)Mn-5Ni austenitic stainless steels. Acta Mater. 45, 2467–2475 (1997).
Ren, Y., Zhao, H., Liu, W. & Yang, K. Effect of cold deformation on pitting corrosion of 00Cr18Mn15Mo2N0.86 stainless steel for coronary stent application. Mater. Sci. Eng. C. 60, 293–297 (2016).
O’Neal, S. L. & Zheng, W. Manganese toxicity upon overexposure: a decade in review. Curr. Environ. Health Rep. 2, 315–328 (2015).
Kim, H., Harrison, F. E., Aschner, M. & Bowman, A. B. Exposing the role of metals in neurological disorders: a focus on manganese. Trends Mol. Med. 28, 555–568 (2022).
Dey, S., Tripathy, B., Kumar, M. S. & Das, A. P. Ecotoxicological consequences of manganese mining pollutants and their biological remediation. Environ. Chem. Ecotoxicol. 5, 55–61 (2023).
Santamaria, A. B. Manganese exposure, essentiality & toxicity. Indian J. Med. Res. 128, 484 (2008).
Patil, A., Kaur, S., Verma, A. & Arya, M. Manganese (Mn 2+) pollution and its bioremediation: an overview. Biochem. Cell. Arch. https://doi.org/10.51470/bca.2024.24.1.1 (2024).
Ding, B., Zheng, P., Ma, P. & Lin, J. Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Adv. Mater. 32, 1905823 (2020).
Huang, P. et al. Manganese-derived biomaterials for tumor diagnosis and therapy. J. Nanobiotechnol. 22, 335 (2024).
Aschner, M., Guilarte, T. R., Schneider, J. S. & Zheng, W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 221, 131–147 (2007).
Yang, C., Wu, H., Gao, X. & Zhang, C. Effect of manganese doping on the bioactivity and antioxidant activity of bioglass. Appl. Phys. A 131, 205 (2025).
Xia, Z. et al. Zebrafish slc30a10 deficiency revealed a novel compensatory mechanism of Atp2c1 in maintaining manganese homeostasis. PLOS Genet 13, e1006892 (2017).
Lu, S. et al. Biodegradable high-nitrogen iron alloy anastomotic staples: in vitro and in vivo studies. Bioact. Mater. 40, 34–46 (2024).
Gao, T., Wang, F., Li, S., Luo, X. & Zhang, K. Manganese regulates manganese-containing superoxide dismutase (MnSOD) expression in the primary broiler myocardial cells. Biol. Trace Elem. Res. 144, 695–704 (2011).
Burlet, E. & Jain, S. K. Manganese supplementation reduces high glucose-induced monocyte adhesion to endothelial cells and endothelial dysfunction in zucker diabetic fatty rats. J. Biol. Chem. 288, 6409–6416 (2013).
Fang, Y. et al. Effects of manganese and iron, alone or in combination, on apoptosis in BV2 cells. Biol. Trace Elem. Res. 202, 2241–2252 (2024).
Peguet, L., Malki, B. & Baroux, B. Influence of cold working on the pitting corrosion resistance of stainless steels. Corros. Sci. 49, 1933–1948 (2007).
Wang, Q., Zhang, B., Ren, Y. & Yang, K. A self-healing stainless steel: role of nitrogen in eliminating detrimental effect of cold working on pitting corrosion resistance. Corros. Sci. 145, 55–66 (2018).
Wang, Q., Zhang, B., Ren, Y. & Yang, K. Eliminating detrimental effect of cold working on pitting corrosion resistance in high nitrogen austenitic stainless steels. Corros. Sci. 123, 351–355 (2017).
Chen, Q. & Thouas, G. A. Metallic implant biomaterials. Mater. Sci. Eng. R. Rep. 87, 1–57 (2015).
Yang, K., Ren, Y. & Wan, P. High nitrogen nickel-free austenitic stainless steel: a promising coronary stent material. Sci. China Technol. Sci. 55, 329–340 (2012).
Feng, H. et al. Effect of nitrogen on corrosion behaviour of a novel high nitrogen medium-entropy alloy CrCoNiN manufactured by pressurized metallurgy. J. Mater. Sci. Technol. 34, 1781 (2018).
Li, J., Yang, Y., Ren, Y., Dong, J. & Yang, K. Effect of cold deformation on corrosion fatigue behavior of nickel-free high nitrogen austenitic stainless steel for coronary stent application. J. Mater. Sci. Technol. 34, 660 (2018).
Leyssens, L., Vinck, B., Van Der Straeten, C., Wuyts, F. & Maes, L. Cobalt toxicity in humans. A review of the potential sources and systemic health effects. Toxicology 387, 43–56 (2017).
Ren, Y., Wan, P., Liu, F., Zhang, B. & Yang, K. In vitro study on a new high nitrogen nickel-free austenitic stainless steel for coronary stents. J. Mater. Sci. Technol. 27, 325–331 (2011).
Shanshan, C. et al. In vivo study on new coronary stents made of high-nitrogen nickel-free stainless steel. Front. Bioeng. Biotechnol. https://doi.org/10.3389/conf.FBIOE.2016.01.02061 (2016).
Chen, S. et al. Assessment of structure integrity, corrosion behavior and microstructure change of AZ31B stent in porcine coronary arteries. J. Mater. Sci. Technol. 39, 39–47 (2020).
Acknowledgements
Q.W. discloses support for the research of this work from the National Natural Science Foundation of China [grant number 52471266] and the National Natural Science Foundation of China [grant number 51801220]. A.L. discloses support for the research of this work from Ningxia key scientific and technological achievements transformation project [grant number 2024CJE09005] and Ningxia Natural Science Foundation Key Project [grant number 2024AAC02066]. L.L. discloses support for the research of this work from Zhejiang Provincial Science and Technology Planning Project [grant number 2024C01151].
Author information
Authors and Affiliations
Contributions
This work was conceived by K.Y. and Q.W. H.L. prepared Figs. 3–8 and wrote the first draft, reviewed by K.Y. and A.L. S.C. prepared Figs. 1–2. L.L. prepared table 1. H.Y. and B.Z. made the analysis of data. Q.W.and A.L.contributed to funding acquisition. K.Y. contributed to project managment. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Liu, H., Chen, S. et al. Nickel free high nitrogen stainless steels with superior corrosion resistance and mechanical properties for coronary stents. npj Mater Degrad 9, 149 (2025). https://doi.org/10.1038/s41529-025-00689-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41529-025-00689-1