Abstract
Deep brain stimulation of the subthalamic nucleus (STN-DBS) is an established intervention for alleviating both motor and non-motor symptoms in advanced Parkinson’s disease (PD). However, patient outcomes may vary widely, underscoring the need for predictive biomarkers. Neuroimaging techniques, such as neurite orientation dispersion and density imaging (NODDI), a biophysical model-based MRI technique, offer promise in forecasting clinical outcomes and supporting preoperative counseling. This prospective, open-label study aimed to identify microstructural markers that correlate with short-term motor outcomes following STN-DBS in PD patients. Thirty-five patients underwent diffusion MRI and comprehensive clinical evaluations preoperatively and six months postoperatively. Evaluations were performed in the ON-medication as well as ON-medication/ON-stimulation state. A whole-brain voxel-wise analysis was conducted to explore associations between microstructural metrics and motor outcomes. Permutation-based statistical methods were applied to adjust for multiple comparisons. Intact microstructure in the bilateral putamen, bilateral insula, and left pallidum was significantly associated with a greater postoperative motor symptom improvement. Additionally, preserved microstructure in the pre- and postcentral gyrus and right precuneus was associated with increased duration with good mobility and without troublesome dyskinesia, and reduced time with poor mobility. These findings suggest that diffusion MRI may serve as valuable tool for identifying patients likely to exhibit favorable motor outcomes following STN-DBS. Incorporating microstructural data into preoperative counseling could enhance patient selection and optimize therapeutic strategies.
Similar content being viewed by others
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus represents an effective intervention for both motor and non-motor symptoms in Parkinson’s disease (PD)1,2,3,4,5. In addition to its local effects at the micro- and mesoscale, it is posited that the efficacy of DBS is derived from its ability to modulate distributed neurophysiological networks6,7. Consequently, the microstructural integrity of the brain regions constituting these networks is believed to be crucial for the therapeutic effectiveness of DBS8. Recent findings have demonstrated that the favorable outcomes related to non-motor symptoms are contingent upon the preservation of microstructural integrity within specific areas of the basal-ganglia-thalamo-cortical circuit8. These outcomes, along with evidence that preoperative levodopa response, tremor-dominant phenotype, baseline frontal score, and off-medication UPDRS part III scores predict short-term motor outcomes9, lend credence to the notion that quantitative MRI techniques, such as neurite orientation dispersion and density imaging (NODDI), may serve a valuable role in preoperative counseling8,10 - a concept that has gained increasing attention in recent years11. NODDI is a sophisticated, multi-compartmental diffusion-weighted MRI technique, employed to investigate the microstructure of brain tissue12. This model yields two voxelwise metrics pertaining to neurite morphology: the neurite density index (NDI), which quantifies the density of axons and dendrites within a voxel, and the orientation dispersion index (ODI), which assesses the degree of neurite dispersion, reflecting their alignment and dendritic arborization12. Importantly, the relationship between NODDI metrics and the underlying tissue properties has recently been corroborated through histological analysis13,14. Furthermore, NODDI models have been demonstrated to possess higher sensitivity compared to conventional DTI models, highlighting their potential utility as clinical tools11. Additionally, NODDI as a supplementary DTI technique exhibits robustness to variations in acquisition parameters with clinically feasible data acquisition times11.
Previous investigations utilizing NODDI in PD have illustrated the model’s capacity to identify disease-related pathology, such as retrograde degeneration of the nigrostriatal pathway11. Furthermore, NODDI metrics have been associated with bimanual motor control, disease severity, and disease duration11,15. The integration of NODDI with conventional DTI metrics in a multi-parametric analysis thus provides complementary insights into microstructural properties and may serve as an imaging-based biomarker for predicting treatment outcomes. Consequently, the present study aims to demonstrate that this approach can discern microstructural properties within brain regions that are associated with motor outcomes after STN-DBS in PD. Specifically, the study concentrates on identifying regions where microstructural metrics correlate with (1) alterations in the overall burden of motor symptoms, as assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS), and (2) variations in periods characterized by good mobility and without troublesome dyskinesia (ON) versus these marked by poor mobility (OFF). The findings of this study could facilitate preoperative patient counseling by identifying specific brain microstructure that predict above- or below-average motor responses to STN-DBS.
Results
Clinical outcomes
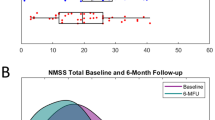
Thirty-five patients with PD (26 males, mean age 58.7 ± 7.4 years) were analyzed. At the six-month follow-up, the following primary outcomes improved: Unified Parkinson’s Disease Rating Scale (UPDRS) part III (p < 0.001, Cohen’s d = 1.02), duration with good mobility and without troublesome dyskinesia (ON, p < 0.001, Cohen’s d = −0.99), and duration with poor mobility (OFF, p < 0.001, Cohen’s d = 0.75). Furthermore, the following scales improved: UPDRS total score (p < 0.001, Cohen’s d = 0.94), UPDRS part I (p = 0.011, Cohen’s d = 0.48), UPDRS part IV (p < 0.001, Cohen’s d = 0.76), Parkinson’s Disease Questionnaire (PDQ)-8 summary index (SI) (p < 0.001, Cohen’s d = 0.69), Levodopa equivalent daily dose (LEDD; p < 0.001, Cohen’s d = 1.3), and LEDD of dopamine agonists (p < 0.001, Cohen’s d = 0.7). Analysis of movement records additionally revealed a reduction of duration with good mobility and troublesome dyskinesia (ON TD) after STN-DBS (p = 0.001, Cohen’s d = 0.55). Longitudinal changes of clinical outcomes are reported in Table 1 and Fig. 1. Longitudinal changes in clinical outcomes of the three- and twelve-month follow-up visits are reported in Supplementary Table 6 and demonstrate consistent effects with those reported above.
Visualization of baseline and 6-month follow-up values of the UPDRS part III (A), time spent with good mobility and no troublesome dyskinesia (ON, B), and time spent with poor mobility (OFF, C). Center line indicates the median, box limits represent upper and lower quartiles and whiskers indicate most extreme data points not considered outliers.
Interaction between fractional anisotropy and postoperative change in motor symptoms
We evaluated the relationship between DTI metrics and postoperative motor symptom change using a generalized linear model corrected for multiple comparisons using a permutation-based approach. Lower regional mean fractional anisotropy values in the anterior division of the right cingulate gyrus, the right inferior fronto-occipital fasciculus, and the left superior longitudinal fasciculus were significantly associated with greater postoperative change in motor symptom burden, as indicated by changes in the UPDRS part III values (negative clusters N1, N2, and N7 cluster-wise p-value (CWP): <0.001–0.035). No clusters demonstrating a positive association between FA and postoperative changes in motor symptoms were identified. This relationship between regional mean fractional anisotropy and motor symptoms is detailed in Supplementary Table 1 and Supplementary Fig. 1.
Interaction between NODDI-parameters and postoperative change in motor symptom burden
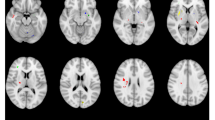
Whole brain analysis of NODDI parameters indicated that both ODI and NDI were significantly correlated with changes in postoperative UPDRS part III scores. These findings are illustrated in Supplementary Tables 2 and 3 as well as Figs. 2 and 3 (ODI) and Supplementary Figs. 3 and 4 (NDI values). Higher ODI values in the left pallidum, bilateral putamen, and bilateral insular cortex were associated with greater change in postoperative motor symptoms (P1-6, CWP: <0.001–0.038, Fig. 2). Conversely, negative associations were exclusively observed in white matter regions adjacent to the left middle frontal gyrus, left cingulate gyrus, and left precuneus (N1-3, CWP: <0.001-0.019, Fig. 3). Furthermore, higher ODI and NDI values in the bilateral insular cortex were related to a greater reduction in postoperative motor symptoms (ODI: P3, P5, CWP: <0.001-.003; NDI: P1–P3, CWP: <0.001–0.032). Additionally, increased NDI values in the left putamen were liked to greater reduction in postoperative motor symptoms (P1, CWP: <0.001). Negative associations between NDI and postoperative motor symptoms were identified in white matter regions adjacent to the left middle frontal gyrus, left precentral gyrus, right precuneus, and left paracingulate gyrus, corresponding to the previously mentioned negative ODI-clusters (N1-3, CWP: <0.001−0.019).
Clusters with a positive association between PD patients’ ODI-values and postoperative change in UPDRS (red), time spent in ON (green), and time spent in OFF (blue), as revealed by the whole brain analysis. Clusterwise P-values were corrected for multiple comparisons using a permutation-based approach and shown as the negative decadic logarithm of the p-value.
Clusters with a negative association between PD patients’ ODI-values and postoperative change in UPDRS (red), time spent in ON (green), and time spent in OFF (blue), as revealed by the whole brain analysis. P-values were corrected for multiple comparisons using a permutation-based approach. Clusterwise P-Values were corrected for multiple comparisons using a permutation-based approach and shown as the negative decadic logarithm of the p-value.
Interaction between microstructural metrics and time with good/poor mobility
Regional mean values of microstructural metrics associated with significant postoperative variations in time spent in the ON and OFF states are presented in Supplementary Tables 4 and 5, as well as Supplementary Figs. 3 and 4. Decreased values of microstructural metrics within gray matter regions of the right pre- and postcentral gyrus and the right precuneus were correlated with increased time spent in the ON state (ODI: N3, N6, N7, CWP: <0.001–0.002). No corresponding negative NDI clusters were identified. Regarding the time spent in the OFF state, associations across extensive gray matter regions were observed. Specifically, higher ODI values in the right cingulate gyrus, left pre- and postcentral gyrus, and the right precuneus were related to greater postoperative changes in time spent in OFF state (ODI: P1-3, CWP: <0.001). Additionally, elevated NDI values in the left middle frontal gyrus and the left precentral gyrus were associated with greater postoperative changes in time spent in the OFF state (NDI: P1, P4, CWP: <0.001–0.01).
Interaction between microstructure and postoperative motor outcomes controlled for medication, age, and disease duration
In a supplementary analysis, we reran the GLM analysis and assessed the relationship between DTI metrics and postoperative motor outcomes (UPDRS-III, time spent in good mobility without troublesome dyskinesia, time spent in poor mobility) while considering LEDD, age, and disease duration as a covariate. For conciseness, findings are displayed in Supplementary Figs. 6–11. While certain smaller clusters and clusters within left pre- and postcentral gyrus associated with poor mobility did not survive correction for multiple comparisons when accounting for the covariates named above, we could replicate the main findings of our analysis for UPDRS part III as well as the time spent in good and poor mobility.
Discussion
In the present study, we employed NODDI-DTI, an innovative method for analyzing diffusion-weighted imaging (DWI) data, to investigate the association between cerebral microstructure and alterations in motor symptoms following subthalamic stimulation in patients with PD. Our findings can be summarized in three key points. First, we demonstrate that intact microstructure within the bilateral putamen, bilateral insula, and left pallidum is associated with a greater reduction in postoperative motor symptoms. Second, we identify specific white matter tracts whose selective degeneration correlates with below-average improvements in postoperative motor symptoms. Third, we find that preserved microstructure in certain brain structures is linked to postoperative increases in the duration of time spent in good mobility and no troublesome dyskinesia, as well as decreases in the time spent in poor mobility.
Parkinson’s disease is characterized by the cardinal symptoms of bradykinesia, rigidity, and tremor, which significantly impair the quality of life of affected individuals16,17. These debilitating symptoms are evaluated using the UPDRS to gauge the overall motor burden18. The UPDRS part III has been established as the standard parameter for overall motor symptom severity in PD and serves as a primary outcome measure in numerous clinical trials19,20,21. In the present study, whole-brain analysis of NODDI parameters identified an association between higher ODI values within bilateral putamen, bilateral insula, and left pallidum and a higher reduction in postoperative UPDRS part III values. Given that ODI is high in gray matter12, this finding supports the hypothesis that the integrity of dendritic processes in these regions is pivotal for achieving beneficial postoperative motor outcomes.
Given the intricate relationship between the underlying disease mechanisms and the physiological effects of the intervention, several aspects have to be considered when interpreting the present findings. First, the pathological accumulation of alpha-synuclein in intraneuronal Lewy inclusions results in neuronal degeneration and significant morphological changes in dendrites22,23. These tissue alterations are not detectable using conventional MRI, whereas NODDI is sensitive to variations in neurite morphology. Previous studies have documented reduced putaminal ODI values in people with PD compared to healthy controls, interpreted as indicative of decreased dendritic length and loss of spines in striatal medium spiny neurons, the primary targets of dopaminergic nigrostriatal projections. Therefore, it can be hypothesized that low ODI values in these structures reflect the compromised neurite morphology described by Kamagata as well as Schröder and colleagues23,24.
Second, given the close topographical relationship between the STN, the putamen, and the pallidum along with the integration of STN in basal ganglia-thalamo-cortical loops, it is essential to examine the putative mechanisms of action of DBS. Besides its effects at the micro- and mesoscale, the high-frequency electrical pulses from DBS electrodes influence interregional networks on a macroscale25. This network modulation has been shown to predict postoperative outcomes across both motor and various non-motor symptoms26. Therefore, it can be postulated that the positive association between ODI and postoperative motor outcomes in the present study reflects the reliance of DBS on intact tissue structure to exert its network effects. In this context, Hermann et al. emphasize the significance of preserved microstructure in motor regions such as the STN, substantia nigra (SN), and putamen for the efficacy of STN-DBS27. Using free interstitial fluid (V-CSF) as a marker of microstructure, the authors identified a correlation between V-CSF-values in their region of interest (ROI) and postoperative improvements in UPDRS part III. Despite notable effect sizes, these results did not reach statistical significance after adjusting for multiple comparisons. In the present study, we address the limitations of region of interest-based and correlation analyses by integrating a whole-brain approach with generalized linear modeling, thus partially confirming the association between intact putaminal microstructure and improvements in motor symptoms. Furthermore, a growing body of literature on structural imaging has identified other anatomical features as predictors of motor outcomes in Parkinson’s disease, including the mesencephalon surface, thalamic volumes, cortical thickness in the left lateral-occipital cortex and morphometry of the superior frontal cortex28,29,30,31. Collectively, these results underline the potential of structural imaging, and microstructural imaging in particular, to evaluate postoperative outcomes of neurostimulation in Parkinson’s disease.
Besides associations with areas of the basal ganglia-thalamo-cortical loop, ODI showed a positive association with post-operative motor burden in the bilateral insular cortex. The insular cortex has been primarily considered a visceral-somatic region within the limbic system32, and is a major target area of thalamocortical projections33,34. Studies suggest a role for the insular cortex in integrating autonomic, cognitive-affective, and somatosensory information, mediating the control of non-motor symptoms in PD35,36. The authors point to the pathological accumulation of alpha-synuclein37 as a cause of altered insular function, subsequently leading to non-motor symptoms such as aberrant sensory processing and urinary issues. Conversely, recent studies have demonstrated an influence of the insular cortex on gating mechanisms in motor control. Wu and colleagues found decreased functional connectivity from the pre-supplementary motor area to the mid-anterior insula in patients with PD38. These disrupted connections indicate a lack of readiness for movement and may partly contribute to the difficulty in initiating movements in PD. Extending this hypothesis, Tinaz et al. proposed a circuit model highlighting the interaction between the insula and the dorsomedial frontal cortex (harboring supplementary motor area (SMA) and pre-SMA) for generating intentional movements39. In this model, the insula processes afferent viscerosensory and somatosensory information from the body and integrates them with motivational and emotional context. This information is conveyed to the frontal cortex, which is involved in various higher-level cognitive functions40,41,42, and is supposed to generate the impetus to move. The authors conclude that altered insular function in PD patients contributes to the difficulty in internally generating intentional movements and in maintaining the speed, size, and vigor of movements39. Regarding the effects of STN-DBS on the insular cortex, Herzog et al. investigated the sensory gating of urinary bladder afferents following STN-DBS43. They demonstrated modulation of activity in the insular cortex and thalamus by STN-DBS, suggesting that partially restoring basal ganglia function via DBS is beneficial for sensory gating and urinary outcomes in PD. Integrating our findings with these studies, we suggest that the positive association between ODI and postoperative motor outcomes reflects the dependency of DBS on intact tissue structure within the bilateral insula to exert beneficial effects on gating processes essential for movement control.
Whole brain analysis of NODDI parameters identified an association between lower FA- and NDI-values in several WM tracts, including the cingulum and a detrimental motor response to STN-DBS. In healthy white matter, NDI is usually high, as fibers are coherent; low NDI values represent axonal loss and degeneration12. Conversely, ODI in white matter is typically low. As most negative FA- and NDI-clusters overlapped with clusters where ODI showed a negative association with postoperative motor symptom burden and lay within regions of high fiber crossing and dispersion, the selective degeneration of crossing fibers might underlie the observed relationship. The results, therefore, support the hypothesis that intact microstructure of the cingulum and its crossing fibers is important for beneficial postoperative changes in motor symptoms.
This study employed whole-brain analysis of NODDI parameters to identify significant associations between favorable motor states and the integrity of microstructural features within the bilateral pre- and postcentral gyri, right precuneus, and cingulum. In particular, an increased duration of time spent in the ON-state associated with preserved microstructural integrity, as indicated by the ODI, in the right pre- and postcentral gyri and the right precuneus. Furthermore, a decrease in the duration of time spent in the OFF-state following STN-DBS was associated with intact microstructure in the right cingulate gyrus, left pre- and postcentral gyri, and right precuneus. Numerous functional MRI (fMRI) studies have shown that neurostimulation significantly impacts the connectivity of the primary motor cortex (for a review see Miao et al. and Li et al.)44,45. These studies provide evidence that STN-DBS alters the resting-state functional connectome, enhancing coupling within the direct pathway while reducing coupling in the hyperdirect pathway. Furthermore, the connectivity between DBS electrodes and a network of brain regions, including the primary motor cortex, is predictive of clinical responses to STN-DBS46. Integrating these studies with the fact that the pre- and postcentral gyrus contain structures essential for voluntary motor control and somatosensory information processing, it is conceivable that intact microstructure within these areas is essential for the efficacy of DBS.
It is important to acknowledge that this research is not without its limitations. First, while the NODDI model is histopathologically validated and widely used in PD research, there are currently no studies validating it specifically in the context of PD. Second, the fundamental principles of the NODDI model may oversimplify the situation, potentially reducing specificity. Third, the resolution of the DTI scan in our study is limited to 2.0 × 2.0 × 2.0 mm³ and 42 diffusion gradients. This resolution and number of gradients, however, is similar to previous NODDI studies47,48,49 and was chosen to find a compromise between scanning time, image resolution, and signal-to-noise ratio, where longer durations in the scanner lead to more noticeable motion artefacts, a crucial consideration in PD. Fourth, the general limitations of open-label studies apply here. While open-label designs have advantages, they also present limitations, including potential placebo effects that may influence subjective measures and patient well-being. Furthermore, subjective measures such as patient diaries can be prone to inaccuracies, as patients may be unable to correctly recognize or report their symptoms. In this context, it is important to note that in the present study, the UPDRS part II, which assesses patients’ motor experiences of daily living, showed no significant difference compared to baseline during the best condition. This result aligns with findings from a large randomized controlled trial, which also reported no significant changes in UPDRS part II scores when assessed under the best condition50. Finally, a control group cannot be included in open-label DBS studies. To mitigate these challenges and reduce bias, several measures were implemented. First, the primary outcomes were selected to include a combination of clinician-rated and patient-reported subjective outcomes. Second, patient-reported outcomes were assessed using standardized instructions and case report forms. Third, the UPDRS part III was evaluated by a single rater blinded to whether the videos were from baseline or follow-up visits, using standardized video recordings. Fourth, assessments from 3- and 12-month follow-up visits demonstrated consistent effects, similar to those observed at the six-month follow-up. Fifth, a complementary analysis accounting for potential confounding variables, including LEDD, age, and disease duration, confirmed the robustness of our findings.
In conclusion, we identified a distinct spatial profile of microstructural alterations associated with motor outcomes following neurostimulation in PD. In particular, intact microstructure within the bilateral putamen, bilateral insula, and left pallidum were associated with a higher reduction in postoperative motor symptoms. Furthermore, preserved microstructure in pre- and postcentral gyrus and right precuneus and cingulum was associated with increases in time spent in ON as well as decreases in time spent in OFF. These findings remain consistent even after accounting for LEDD, age, and disease duration. Our results highlight the possibility of preoperatively assessing microstructural alterations to support patient counseling and treatment planning by identifying patients likely to experience above- or below-average motor responses.
Methods
Participants
In this ongoing observational study, thirty-seven PD patients were enrolled upon written informed consent. Inclusion criteria comprised indication for DBS surgery due to advanced PD following international criteria, including the presence of motor fluctuations and/or dyskinesias51. Patients with pathological MRI, concurrent neurological or psychiatric conditions, or impaired visual or auditory function were excluded. Two patients were excluded due to missing follow-up data. The present study includes a cohort of participants that partially overlaps with those reported in our previous publication, as part of the ongoing observational study. The study was conducted according to the Declaration of Helsinki and approved by the Philipps-University of Marburg ethics committee (study-number: 155/17).
Clinical assessment
Patients were evaluated at the preoperative baseline and at six months after DBS lead surgery. Both study visits were conducted while the patients were in the ON-medication state, whereas follow-up assessments were performed in the ON-medication/ON-stimulation state (MedON/StimON). Neurosurgical standards were defined by the involved neurosurgeons to guarantee an optimal approach and were consistent with the methodology described by Deuschl et al.52. Standardized case report forms were utilized to gather demographic and clinical data, including the UPDRS, the PDQ-8, and overall mobility. The Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) is a clinician-rated scale evaluating motor symptom severity ranging from 0 (no impairment) to 108 (maximum impairment)18. The UPDRS-III was evaluated by a single rater (PAL) based on standardized video recordings, except for the rigidity items, which cannot be evaluated via video analysis. The rater was blinded to whether the videos originated from baseline or follow-up visits. The PDQ-8 is a validated tool for assessing quality of life across eight dimensions in PD patients undergoing DBS surgery. It provides a summary index (SI) score ranging from 0 (no impairment) to 100 (maximum impairment)53. Mobility data were recorded in a patient diary, documenting the number of hours per day spent in good mobility and without troublesome dyskinesia (ON), poor mobility (OFF), good mobility with troublesome dyskinesia (ON TD), and sleep (SLEEP)54. Patients were instructed to complete these diaries for the 36 h preceding the study visit. The LEDD was calculated according to the methodology described by Jost et al.55. To ensure a consistent postoperative adjustment of medication and mitigate a potential confounding effect of LEDD on motor outcomes, we implemented a standardized protocol for reducing LEDD after DBS implantation, aligning with the approach used in the EARLYSTIM study52. Likewise, the adjustment of stimulation parameters was performed using a standardized protocol, also based on the methodology employed in the EARLYSTIM study52. Demographic and clinical characteristics are summarized in Table 1 and Fig. 1.
To increase the reliability of our clinical data and minimize the potential influence of symptom fluctuations caused by varying stressors, we performed a supplementary analysis incorporating data from the three- and twelve-month follow-up visits.
MRI data acquisition and processing
At preoperative baseline, PD patients were scanned at the University of Marburg’s Core Unit Brain Imaging using a 3-Tesla Trio scanner (Siemens, Erlangen, Germany). During image acquisition, patients were awake and in the ON-medication state. The acquisition protocol comprised the following sequences:
-
1.
3D T1-weighted Magnetization Prepared - RApid Gradient Echo sequence (MPRAGE, field of view (FoV) = 256 mm, matrix 256 × 256, 176 slices, slice thickness 1 mm, voxel dimension 1.0 × 1.0 × 1.0 mm³, repetition time (TR) = 1900 ms, echo time (TE) = 2.26 ms, inversion time (TI) = 900 ms, flip-angle = 9°, bandwidth (BW) = 200 Hz/Pixel, parallel imaging (GRAPPA) with factor 2).
-
2.
diffusion weighted imaging (DWI) (FoV = 256 mm, matrix 128 × 128, slice thickness 2 mm, distance factor 0%, voxel dimension 2.0 × 2.0 × 2.0 mm³, TR = 7900 ms, TE = 90 ms, BW = 1502 Hz/Pixel, 42 diffusion encoding gradients, three intermittent non-weighted b0 images (b = 0 s/mm²), high b-value b = 1000 s/mm², GRAPPA with factor 2).
All images were investigated to be free of motion or ghosting and high frequency and/or wrap-around artefacts at the time of image acquisition. Postoperative evaluation included a computed tomography (CT) scan to assess for complications such as intracranial bleeding, edema, or lead misplacement. No postoperative complications were identified on imaging.
Image Processing
Analysis of structural images was performed within the FreeSurfer image analysis suite version 7.1.1 (http://surfer.nmr.mgh.harvard.edu), as previously reported by our research group15. The processing pipeline comprised several steps including skull stripping, automated Talairach transformation, cortical and subcortical segmentation, intensity normalization, tessellation of the gray/white matter boundary, automated topology correction, and surface deformation based on intensity gradients.
The analysis of diffusion-weighted images was carried out within the FMRIB Software Library (FSL) analysis suite version 6.0.5.2 (https://fsl.fmrib.ox.ac.uk/fsl). Raw DWI volumes were registered and resampled to the first b0 volume to correct for eddy-current distortions and involuntary movements. Subsequently, the diffusion tensor for each voxel was estimated using linear regression from which FA was derived. Additionally, NODDI-DTI, a modification of NODDI, was employed to obtain NDI and ODI from the DTI data. Visual inspection of the b0 images confirmed that no alterations beyond those inherent in the tissue structure contributed to the observed effects. Regional analyses were performed by linearly registering the first b0 image of each scan to the structural T1-weighted image using a boundary-based registration method and resulting in an affine matrix. By applying the inverse of this matrix, the T1-derived segmentations and brain masks were transformed into diffusion space. With the aid of both linear and nonlinear transformations, FA-maps were initially co-registered to the MNI152 standard space56. Subsequently, masked FA-, NDI-, and ODI-maps were registered to the MNI152 space using the transformation established in the preceding step, excluding voxels of brain tissue that were not consistently present across all subjects from the analysis.
To verify the correct placement of the electrodes, we performed a supplementary analysis using the Lead-DBS toolbox with default parameters (www.lead-dbs.org). Briefly, advanced normalization tools (ANTs, http://stnava.github.io/ANTs/) were employed to linearly coregister postoperative CT images with preoperative MRI. The images were then nonlinearly normalized to standard space (ICBM 2009b NLIN, Asym), and the PaCER algorithm was utilized for electrode reconstruction (Supplementary Fig. 5). This approach allowed us to confirm accurate lead placement in the participants.
Statistical analysis
Statistical analysis of clinical outcomes was conducted utilizing MATLAB R2020b (The MathWorks, Inc.). Mean changes in clinical scores between baseline and the 6-month follow-up (6-MFU) were evaluated using Wilcoxon signed-rank-test or t-tests, according to results of the Shapiro-Wilk test for normality assessment. The false discovery rate was controlled by employing the Benjamini-Hochberg method, and effect sizes were calculated in accordance with Cohen’s conventions57,58. Reported p-values are two-sided and were deemed significant at p < 0.05.
Statistical voxelwise analysis of imaging data was performed using the tool mri_glmfit of FreeSurfer. In this analysis, clinical outcomes were represented as percentage difference in UPDRS-III scores, time spent in good mobility without troublesome dyskinesia, and time spent in poor mobility. Associations between microstructure and time spent in good mobility with troublesome dyskinesia (ON TD) were not performed due to the small number of patients experiencing dyskinesia (n = 14).
Associations between metrics of microstructure and changes in clinical outcomes were conducted across the whole brain using a generalized linear model59. A permutation-based approach utilizing the Analysis of Functional NeuroImages (AFNI) null-z simulator, was employed to correct for multiple comparisons using 12,000 simulations under the null hypothesis60. Clusters were formed using a threshold of p < 0.01, and a clusterwise p-value was calculated. Results were accepted as significant when the clusterwise p < 0.05. In a complementary analysis, we reran the statistical voxelwise analysis incorporating LEDD, age, and disease duration as a covariate (c.f. Supplementary Material).
Acknowledgement
The authors would like to thank the participants for their active engagement in this study.
Data availability
The data that support the findings of this study is available on request from the corresponding author (PAL). The data is not publicly available due to privacy or ethical restrictions. All tools used for the analysis of MRI data are based on FreeSurfer Version 7.1 (http://surfer.nmr.mgh.harvard.edu/) and FSL 6.0.5.2 (http://www.fmrib.ox.ac.uk/fsl) packages, which are freely available. Scripts for automation were written in tcshell and parts of the statistics were written in Python using the packages numpy, pandas, seaborn, matplotlib, nibabel and scipy, which are also freely available. Python program code for the analysis of NODDI-DTI is available from https://github.com/dicemt/DTI-NODDI.
Code availability
The data that support the findings of this study is available on request from the corresponding author (PAL). The data is not publicly available due to privacy or ethical restrictions. All tools used for the analysis of MRI data are based on FreeSurfer Version 7.1 (http://surfer.nmr.mgh.harvard.edu/) and FSL 6.0.5.2 (http://www.fmrib.ox.ac.uk/fsl) packages, which are freely available. Scripts for automation were written in tcshell and parts of the statistics were written in Python using the packages numpy, pandas, seaborn, matplotlib, nibabel and scipy, which are also freely available. Python program code for the analysis of NODDI-DTI is available from https://github.com/dicemt/DTI-NODDI.
Change history
30 June 2025
This article has been updated to amend the license information.
Abbreviations
- 6-MFU:
-
6-month follow-up
- LEDD:
-
Levodopa equivalent daily dose
- LEDD-DA:
-
LEDD of Dopamine Agonists
- M:
-
mean
- OFF:
-
time spent in poor mobility
- ON:
-
time spent in good mobility and without troublesome dyskinesia
- ON TD:
-
time spent in good mobility with troublesome dyskinesia
- PDQ-8 SI:
-
8-item Parkinson’s Disease Questionnaire summary index
- SD:
-
standard deviation
- SLEEP:
-
time allocated to sleep
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
References
Deuschl, G. et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355, 896–908, https://doi.org/10.1056/NEJMoa060281 (2006).
Jost, S. T. et al. Non-motor effects of deep brain stimulation in Parkinson’s disease motor subtypes. Parkinsonism Relat. Disord. 109, 105318, https://doi.org/10.1016/j.parkreldis.2023.105318 (2023).
Jost, S. T. et al. A prospective, controlled study of non-motor effects of subthalamic stimulation in Parkinson’s disease: results at the 36-month follow-up. J. Neurol. Neurosurg.Psychiatry 91, 687–694, https://doi.org/10.1136/jnnp-2019-322614 (2020).
Gronostay, A. et al. Stratifying quality of life outcome in subthalamic stimulation for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2023-332272 (2023).
Sauerbier, A. et al. Predictors of short-term anxiety outcome in subthalamic stimulation for Parkinson’s disease. NPJ Parkinson’s. Dis. 10, 114, https://doi.org/10.1038/s41531-024-00701-6 (2024).
Albano, L. et al. Altered Functional Connectivity of the Subthalamic Nucleus in Parkinson’s Disease: Focus on Candidates for Deep Brain Stimulation. J. Parkinsons Dis. 13, 797–809, https://doi.org/10.3233/JPD-230005 (2023).
Petry-Schmelzer, J. N. et al. Non-motor outcomes depend on location of neurostimulation in Parkinson’s disease. Brain J. Neurol. 142, 3592–3604, https://doi.org/10.1093/brain/awz285 (2019).
Loehrer, P. A. et al. Microstructure predicts non-motor outcomes following deep brain stimulation in Parkinson’s disease. NPJ Parkinson’s. Dis. 10, 104, https://doi.org/10.1038/s41531-024-00717-y (2024).
Cavallieri, F. et al. Predictors of long-term outcome of subthalamic stimulation in Parkinson Disease. Ann. Neurol. 89, 587–597, https://doi.org/10.1002/ana.25994 (2021).
Loehrer, P. A. et al. No evidence for an association of voxel-based morphometry with short-term non-motor outcomes in deep brain stimulation for Parkinson’s disease. NPJ Parkinson’s. Dis. 10, 91, https://doi.org/10.1038/s41531-024-00695-1 (2024).
Kamiya, K., Hori, M. & Aoki, S. NODDI in clinical research. J. Neurosci. Methods 346, 108908, https://doi.org/10.1016/j.jneumeth.2020.108908 (2020).
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016, https://doi.org/10.1016/j.neuroimage.2012.03.072 (2012).
Grussu, F. et al. Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology. Ann. Clin. Transl. Neurol. 4, 663–679, https://doi.org/10.1002/acn3.445 (2017).
Sato, K. et al. Understanding microstructure of the brain by comparison of neurite orientation dispersion and density imaging (NODDI) with transparent mouse brain. Acta Radio. Open 6, 2058460117703816, https://doi.org/10.1177/2058460117703816 (2017).
Loehrer, P. A. et al. Microstructural alterations predict impaired bimanual control in Parkinson’s disease. Brain Commun. 4, fcac137, https://doi.org/10.1093/braincomms/fcac137 (2022).
Chaudhuri, K R & Fung, V S C Fast Facts: Parkinson’s Disease (Health Press Limited, 2016).
Calvano, A., Timmermann, L., Loehrer, P. A., Oehrn, C. R. & Weber, I. Binaural acoustic stimulation in patients with Parkinson’s disease. Front. Neurol. 14, 1167006, https://doi.org/10.3389/fneur.2023.1167006 (2023).
Fahn, S. Unified Parkinson’s disease rating scale. Recent Developments in Parkinson’s Disease, 153–163 (1987).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Jost, S. T. et al. Gender gap in deep brain stimulation for Parkinson’s disease. NPJ Parkinson’s. Dis. 8, 47, https://doi.org/10.1038/s41531-022-00305-y (2022).
Sauerbier, A. et al. The new Satisfaction with Life and Treatment Scale (SLTS-7) in patients with Parkinson’s disease. J. Parkinsons Dis, https://doi.org/10.3233/jpd-212823 (2021).
Halliday, G. M., Leverenz, J. B., Schneider, J. S. & Adler, C. H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 29, 634–650, https://doi.org/10.1002/mds.25857 (2014).
Kamagata, K. et al. Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur. Radiol. 26, 2567–2577, https://doi.org/10.1007/s00330-015-4066-8 (2016).
Schroter, N. et al. Disentangling nigral and putaminal contribution to motor impairment and levodopa response in Parkinson’s disease. NPJ Parkinson’s. Dis. 8, 132, https://doi.org/10.1038/s41531-022-00401-z (2022).
Horn, A. Connectomic Deep Brain Stimulation (2022).
Chu, C. et al. Subthalamic and pallidal stimulation in Parkinson’s disease induce distinct brain topological reconstruction. NeuroImage 255, 119196, https://doi.org/10.1016/j.neuroimage.2022.119196 (2022).
Hermann, M. G. et al. The connection of motor improvement after deep brain stimulation in Parkinson’s disease and microstructural integrity of the substantia nigra and subthalamic nucleus. NeuroImage. Clin. 42, 103607, https://doi.org/10.1016/j.nicl.2024.103607 (2024).
Bonneville, F. et al. Parkinson disease, brain volumes, and subthalamic nucleus stimulation. Neurology 64, 1598–1604, https://doi.org/10.1212/01.wnl.0000160401.24880.83 (2005).
Younce, J. R., Campbell, M. C., Perlmutter, J. S. & Norris, S. A. Thalamic and ventricular volumes predict motor response to deep brain stimulation for Parkinson’s disease. Parkinsonism Relat. Disord. 61, 64–69, https://doi.org/10.1016/j.parkreldis.2018.11.026 (2019).
Frizon, L. A. et al. Cortical thickness in visuo-motor areas is related to motor outcomes after STN DBS for Parkinson’s disease. Parkinsonism Relat. Disord. 71, 17–22, https://doi.org/10.1016/j.parkreldis.2020.01.006 (2020).
Muthuraman, M. et al. Effects of DBS in parkinsonian patients depend on the structural integrity of frontal cortex. Sci. Rep. 7, 43571, https://doi.org/10.1038/srep43571 (2017).
Uddin, L. Q., Nomi, J. S., Hebert-Seropian, B., Ghaziri, J. & Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 34, 300–306, https://doi.org/10.1097/WNP.0000000000000377 (2017).
Allen, G. V., Saper, C. B., Hurley, K. M. & Cechetto, D. F. Organization of visceral and limbic connections in the insular cortex of the rat. J. Comp. Neurol. 311, 1–16 (1991).
Clasca, F., Llamas, A. & Reinoso-Suarez, F. Insular cortex and neighboring fields in the cat: a redefinition based on cortical microarchitecture and connections with the thalamus. J. Comp. Neurol. 384, 456–482 (1997).
Christopher, L., Koshimori, Y., Lang, A. E., Criaud, M. & Strafella, A. P. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 137, 2143–2154, https://doi.org/10.1093/brain/awu084 (2014).
Sauerbier, A. et al. Predictors of short-term impulsive and compulsive behaviour after subthalamic stimulation in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 92, 1313–1318, https://doi.org/10.1136/jnnp-2021-326131 (2021).
Braak, H., Ghebremedhin, E., Rüb, U., Bratzke, H. & Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318, 121–134, https://doi.org/10.1007/s00441-004-0956-9 (2004).
Wu, T. et al. Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum. Brain Mapp. 32, 1443–1457, https://doi.org/10.1002/hbm.21118 (2011).
Tinaz, S. et al. Insula as the interface between body awareness and movement: a neurofeedback-guided kinesthetic motor imagery study in Parkinson’s disease. Front. Hum. Neurosci. 12, 400054, https://doi.org/10.3389/FNHUM.2018.00496/BIBTEX (2018).
Loehrer, P. A. et al. Ageing changes effective connectivity of motor networks during bimanual finger coordination. NeuroImage 143, 325–342, https://doi.org/10.1016/j.neuroimage.2016.09.014 (2016).
Loehrer, P. A. et al. Increased prefrontal top-down control in older adults predicts motor performance and age-group association. NeuroImage 240, 118383, https://doi.org/10.1016/j.neuroimage.2021.118383 (2021).
Nettersheim, F. S. et al. Dopamine substitution alters effective connectivity of cortical prefrontal, premotor, and motor regions during complex bimanual finger movements in Parkinson’s disease. NeuroImage 190, 118–132, https://doi.org/10.1016/j.neuroimage.2018.04.030 (2019).
Herzog, J. et al. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain 131, 132–145, https://doi.org/10.1093/brain/awm254 (2008).
Li, Z. et al. BOLD frequency-dependent alterations in resting-state functional connectivity by pallidal deep brain stimulation in patients with Parkinson’s disease. J. Neurosurg. 139, 1354–1365, https://doi.org/10.3171/2023.1.JNS221858 (2023).
Miao, J. et al. Use of functional MRI in deep brain stimulation in Parkinson’s diseases: a systematic review. Front. Neurol. 13, 849918, https://doi.org/10.3389/fneur.2022.849918 (2022).
Horn, A. et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 82, 67–78, https://doi.org/10.1002/ana.24974 (2017).
Kamagata, K. et al. Gray matter abnormalities in idiopathic Parkinson’s disease: evaluation by diffusional kurtosis imaging and neurite orientation dispersion and density imaging. Hum. Brain Mapp. 38, 3704–3722, https://doi.org/10.1002/hbm.23628 (2017).
Ogawa, T. et al. White matter and nigral alterations in multiple system atrophy-parkinsonian type. NPJ Parkinson’s. Dis. 7, 96, https://doi.org/10.1038/s41531-021-00236-0 (2021).
Mitchell, T. et al. Neurite orientation dispersion and density imaging (NODDI) and free-water imaging in Parkinsonism. Hum. Brain Mapp. 40, 5094–5107, https://doi.org/10.1002/hbm.24760 (2019).
Schuepbach, W. M. et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 368, 610–622, https://doi.org/10.1056/NEJMoa1205158 (2013).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601, https://doi.org/10.1002/mds.26424 (2015).
Deuschl, G. et al. Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson’s disease: Concept and standards of the EARLYSTIM-study. Parkinsonism Relat. Disord. 19, 56–61, https://doi.org/10.1016/j.parkreldis.2012.07.004 (2013).
Jenkinson, C., Fitzpatrick, R., Peto, V., Greenhall, R. & Hyman, N. The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychol. Health 12, 805–814 (1997).
Hauser, R. A. et al. A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin. Neuropharmacol. 23, 75–81, https://doi.org/10.1097/00002826-200003000-00003 (2000).
Jost, S. T. et al. Levodopa dose equivalency in Parkinson’s disease: updated systematic review and proposals. Movement Disord. https://doi.org/10.1002/mds.29410 (2023).
Andersson, J. L. R., Jenkinson, M & Smith, S (2007).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.: Ser. B (Methodol.) 57, 289–300 (1995).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (L. Erlbaum Associates, 1988).
Belke, M. et al. Diffusion Tensor Imaging (DTI) in idiopathic REM sleep behaviour disorder (iRBD). Klinische Neurophysiol. 41, ID136 (2010).
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25, https://doi.org/10.1002/hbm.1058 (2002).
Acknowledgements
The authors would like to thank the participants for their active engagement in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
P.A.L.: study concept and design, data acquisition, data analysis, drafting of the manuscript. J.F.: data acquisition, drafting of the manuscript. M.Bo: data acquisition, surgical intervention, critical revision of the manuscript. A.C.: data acquisition, critical revision of the manuscript. H.S.D.: study design, critical revision of the manuscript. J.W.: data acquisition, critical revision of the manuscript. A.S. data acquisition, critical revision of the manuscript. CA: data acquisition, critical revision of the manuscript. SK: critical revision of the manuscript. C.N.: data acquisition, surgical intervention, critical revision of the manuscript. L.T.: study design, critical revision of the manuscript. M.Be. study concept and design, data acquisition, data analysis, drafting of the manuscript. D.J.P.: study concept and design, data acquisition, data analysis, drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.A.L. was supported by the SUCCESS-Program of the Philipps-University of Marburg, the ‘Stiftung zur Förderung junger Neurowissenschaftler’, and the Professor Klaus Thiemann Foundation in the German Society of Neurology. J.F. reports no financial disclosures. M.Bo is a scientific consultant for Brainlab. A.C. has participated in a training course which was industry-funded by Stada Arzneimittel A.G. H.S.D. was funded by the EU Joint Program—Neurodegenerative Disease Research (JPND), the Prof. Klaus Thiemann Foundation in the German Society of Neurology, the Felgenhauer Foundation, the KoelnFortune program of the Medical Faculty of the University of Cologne and has received honoraria by Everpharma, Kyowa Kirin, Bial, Oruen, and Stadapharm. J.W. reports no financial disclosures. AS reports no financial disclosures. C.A. reports no financial disclosures. S.K. reports no financial disclosures. C.N. is a scientific consultant for Brainlab. L.T. received payments as a consultant for Medtronic Inc. and Boston Scientific and received honoraria as a speaker on symposia sponsored by Bial, Zambon Pharma, UCB Schwarz Pharma, Desitin Pharma, Medtronic, Boston Scientific, and Abbott. The institution of L.T., not L.T. personally, received funding from the German Research Foundation, the German Ministry of Education and Research, and Deutsche Parkinson Vereinigung. M.Be reports no financial disclosures. DJP has received honoraria for speaking at symposia sponsored by Boston Scientific Corp, Medtronic, AbbVie Inc, Zambon and Esteve Pharmaceuticals GmbH. He has received honoraria as a consultant for Boston Scientific Corp and Bayer, and he has received a grant from Boston Scientific Corp for a project entitled “Sensor-based optimization of Deep Brain Stimulation settings in Parkinson’s disease” (COMPARE-DBS). The institution of DJP, not DJP personally, has received funding from the German Research Foundation, the German Ministry of Education and Research, the International Parkinson Foundation, the Horizon 2020 program of the EU Commission, and the Pohl Foundation in Marburg. Finally, DJP has received travel grants to attend congresses from Esteve Pharmaceuticals GmbH and Boston Scientific Corp.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loehrer, P.A., Freigang, J., Bopp, M.H.A. et al. Microstructure is associated with motor outcomes following Deep Brain Stimulation in Parkinson’s disease. npj Parkinsons Dis. 11, 81 (2025). https://doi.org/10.1038/s41531-025-00930-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-00930-3