Abstract
Many studies have reported increased mortality risk in patients with Parkinson’s disease (PD), but few have investigated the risk factors for PD mortality, including medical and socioeconomic factors. We applied an explainable artificial intelligence (XAI) model to predict long-term all-cause mortality in patients with PD using administrative healthcare data collected at PD diagnosis. Among seven machine learning algorithms, XGBoost achieved the best performance (10-year area under the receiver operating characteristic curve (AUROC): 0.836; 5-year AUROC: 0.894). The most important contributing feature to PD mortality was age, followed by male sex and pneumonia. Using XAI models, the nonlinear association between contributing factors and PD mortality was assessed, and an optimal target value to reduce mortality was found. In addition, prediction of individualized 10-year mortality risk for each PD participant was possible. Our XAI modeling pipeline demonstrated the feasibility to predict long-term mortality in patients with PD using preexisting healthcare data.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, second only to Alzheimer’s disease (AD). In the increasingly aging society of many countries, the prevalence of PD is increasing, which increases the socioeconomic burden on both individuals and society1. Although PD is characterized by cardinal motor symptoms, including bradykinesia, resting tremor, rigidity, and postural instability, various non-motor symptoms may also appear during the prodromal stage or course of the disease2. Non-motor symptoms include autonomic dysfunction, psychiatric problems, and cognitive impairment, which have a significant impact on disease progression and quality of life2,3. As such, PD has recently been recognized as a multisystemic disease that encompasses both non-motor and motor symptoms.
Many previous studies have investigated the mortality risk associated with PD, suggesting an overall 2-3 fold increased risk of mortality in PD patients compared to the general population1,4. However, the number of studies focusing on the risk factors for PD mortality is relatively small. The characteristics of motor or non-motor symptoms of PD have mainly been investigated in relation to mortality, with results suggesting that severe motor symptoms, absence of asymmetry, postural instability gait disturbance type, and cognitive impairment increase the mortality of PD5,6,7. The association between mortality and factors such as socioeconomic factors and comorbidity has rarely been investigated in previous studies4,8,9. PD usually occurs in older people, and older people are more likely to present with a variety of comorbidities. We hypothesized that these non-PD-specific medical and sociodemographic factors could have complex associations with mortality risk and may influence mortality risk in individuals with PD.
Recently, artificial intelligence (AI) has begun to play an important role in the medical field10,11,12. Among AI methods, machine learning (ML) can be used to extract knowledge from large amounts of data, allowing the prediction of outcomes; as such, ML has been used to predict the occurrence, prognosis, or survival of various diseases11,13. However, the black box nature of AI systems makes it difficult to understand how the AI system arrives at its prediction14. Therefore, explainable Artificial Intelligence (XAI) methods are currently emerging as a technology to understand the “why and how” of the outcomes of AI models14,15,16. The explainability of predictions is very important for clinical applications in clinical practice, especially for medical AI models. Therefore, in this study, first, we applied ML models to predict the 5- and 10-year all-cause mortality risks of PD using data from the Korean National Health Insurance Service (NHIS). Then, for explainability, we computed the SHapley Additive exPlanations (SHAP) values for mortality prediction model with best performance.
The primary aim of this study was to apply and compare XAI models to predict long-term all-cause mortality in patients with PD using healthcare data at the time of PD diagnosis. Second, using XAI, we sought to identify the risk and protective factors for all-cause mortality in PD and to understand the complex association between these factors and mortality. In addition, we investigated the feasibility of using administrative healthcare data to predict the individualized 10-year mortality risk for each PD participant. In this study, we attempted to predict the mortality of PD by considering a wide range of variables including non-PD-specific factors that have shown associations with PD mortality. We also attempted to find the unrecognized risk factors for PD mortality applying AI algorithms.
Results
Participant characteristics
A total of 36,480 individuals with PD were enrolled, and 165 features were used to build the mortality prediction model. Table 1 presents the demographic and medical characteristics of patients with PD who died within 10 years and those who did not. There were significant differences in sex and age between the two cohorts; there was a higher proportion of males and the mean age was ~7 years higher in patients with PD who died compared to those who did not.
Patients with PD who died tended to smoke and were less likely to perform regular physical activity (PA) compared to those who did not. The body mass index (BMI) was also lower in the PD patients who died. The mean values of blood pressure and fasting blood sugar were higher in PD patients who died, and the mean values of total cholesterol and hemoglobin (Hb) were lower than those who did not. Patients with PD who died also had a higher prevalence of 43 out of 150 comorbidities included in the study compared to those who did not, including hypertension, type 2 diabetes mellitus, ischemic infarction, dementia, including AD, pneumonia, and neurogenic bladder (Supplementary Table 1).
Evaluation of predictive performance among AI models
The performance of the following three prediction models according to different risk factors for PD mortality is presented in Table 2: Model 1 included 15 variables, such as socioeconomic status, lifestyle factors, anthropometric data, and laboratory finding; Model 2 included the top 20 most frequent comorbidities in addition to model 1; and Model 3 included the top 150 most frequent comorbidities in addition to model 1. Model performance generally increased as more variables were included in the models, with Model 3 showing the best performance. Therefore, in this study, we used Model 3 to predict 10-year and 5-year mortality after PD. XGBoost showed the highest accuracy, and the area under the receiver operating characteristic curve (AUROC) values for predicting 10-year and 5-year mortality using XGBoost were 0.836 (95% CI 0.827-0.845) and 0.894 (95% CI 0.886–0.902), respectively (Fig. 1, Supplementary Fig. 1). Supplementary Tables 2, 3 present the other detailed performance metrics. Given that the accuracy in predicting mortality appeared to be similar between the Gradient Boosting and XGBoost models, we used DeLong’s test to determine the difference in AUROCs between the two AI models, finding that XGBoost had a significantly better accuracy in predicting mortality than Gradient Boosting (10-year mortality XGBoost vs. Gradient Boosting, p < 0.01; 5-year mortality XGBoost vs. Gradient Boosting, p < 0.01).
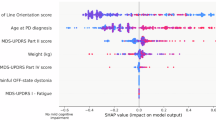
Each point represents a SHAP value for a single participant. The colors indicate the feature values, with purple denoting higher values and yellow denoting lower values. The features are ranked in descending order of importance on the y-axis. The plot highlights the 20 most significant predictors of 10-year mortality after PD, including age, sex, comorbidities, body mass index, and hemoglobin level.
Explainable AI for mortality prediction in PD patients
We computed the SHAP values for mortality prediction model of patients with PD using the XGBoost model, which demonstrated the best performance. Figure 2 presents a SHAP summary plot that explicitly shows the strength and direction of the top 20 most impactful characteristics for predicting the 10-year mortality. The top 20 factors included age and sex as sociodemographic variables, BMI as an anthropometric variable, fasting glucose and Hb levels as laboratory variables, and co-morbidities. Among these factors, age contributed the most to the prediction of mortality in patients with PD, followed by sex, pneumonia, AD, dyslipidemia, BMI, and ischemic infarction. Older age, male sex, pneumonia, dementia, including AD, ischemic infarction, the absence of dyslipidemia, and a low BMI score had a greater impact on predicting mortality.
The x-axis represents the value of each feature, and the y-axis indicates the SHAP value, reflecting the influence of changes in each feature on the SHAP value for the predicted risk of mortality. The color intensity corresponds to the distribution of each feature, demonstrating how the prediction changes with the feature values.
We further predicted the 5-year mortality of patients with PD. Supplementary Fig. 2 presents a SHAP summary chart for the 5-year mortality prediction model. Similar to the 10-year mortality prediction model, age contributed the most to the prediction of mortality in patients with PD, followed by sex and pneumonia. However, in the 5-year prediction model, laboratory values, including Hb and glucose, and anthropometric values, including BMI and blood pressure, were highly ranked compared to the 10-year mortality prediction model. Regarding comorbidities, brain diseases such as ischemic infarction, traumatic brain injury, and dementia had a lower impact on the 5-year than the 10-year prediction model.
Supplementary Fig. 3 presents a SHAP summary plot of age-stratified 10-year mortality prediction model. In PD patients < 70 years, age was the most important predictive factor, whereas sex contributed the most to mortality prediction in PD patients ≥ 70 years. Brain diseases and depression were more highly ranked as predictive factors in PD patients < 70 years compared to those ≥ 70 years.
Using the SHAP dependence plot, we monitored how the changes in each of the top 20 variables contributed to the XGBoost model output (Fig. 2). The scatter plot of the blue dots shows the SHAP values according to the changes in each variable, for which SHAP values exceeding zero represent an increased risk of mortality. Our analysis showed that the SHAP value increased with age, exceeding zero in patients aged approximately > 70 years. The SHAP value decreased as BMI increased to approximately 25, plateauing thereafter, suggesting a decreased mortality risk in PD patients with a BMI greater than about 25. Regarding laboratory variables, an Hb level of 15 and fasting glucose level of 100 mg/dL showed the lowest SHAP value.
Each plot represents the prediction for a different individual (persons A, B, and C), with the x-axis indicating the impact of the SHAP value on the model output. Features pushing the prediction higher (towards an increased risk of mortality) are shown in red, whereas those pushing the prediction lower (towards a decreased risk) are shown in yellow. For each individual, the key features influencing mortality prediction are listed in the order of impact. The length and color intensity of each bar denote the magnitude and direction of the effect of the feature on an individual’s mortality risk. “1” indicates the presence, and “0” indicates the absence of a feature.
The SHAP interaction plot displays how the effect of one feature on the outcome differs across the strata of another feature, highlighting the complex relationship between the two features and the outcome in the XGBoost model. Supplementary Fig. 4 presents the interaction plots of (a) age, (b) sex, (c) pneumonia, (d) AD, and (e) BMI with other variables. The yellow scatterplot represents the contribution in the positive direction, while purple represents the contribution in the negative direction. The association between age and mortality in PD differed according to other features (Supplementary Fig. 4(a)). Regarding the interaction between age and sex, although mortality risk increased with age, the effects of age on PD mortality were more prominent in males than in females. The increased mortality risk associated with low BMI and Hb levels was more pronounced in older patients. Regarding comorbidities, the increased mortality in relation to age was clearer in patients with pneumonia, dementia, and ischemic infarction than in those without. In contrast, the age-related increase in mortality was less significant in patients with dyslipidemia than in those without. On the other hand, there was no definite interaction between age and other comorbidities, such as gastroesophageal reflux disease, dyspepsia, and shoulder disease on mortality in patients with PD.
Figure 4 shows the SHAP force plots for three randomly selected instances from the actual estimation results. The plots depict the default values (0.188), representing the average of the model estimation results for the training set, for which each prediction begins with this value. Red arrows indicate the extent to which the input variables increased mortality, whereas yellow arrows represent the influence of input variables that suppressed mortality (increased survival). The magnitude of the area occupied by each arrow indicates the degree of influence of each variable. In person A, despite the positive effects of female sex, age, and other comorbidities on survival, the presence of dementia contributed greatly on mortality, leading to a prediction of death. In person B, despite the male sex and the presence of ischemic infarction, the absence of pneumonia and AD, combined with a relatively young age of 57 years, decreased the risk of mortality, resulting in a prediction of survival. In person C, advanced age, pneumonia, AD, and male sex significantly contributed to increased mortality, resulting in a high mortality risk.
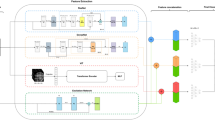
Depiction of the process followed to apply an AI model to predict mortality among Parkinson’s disease from Korean NHIS database, including 36,480 individuals. The initial steps involved data preparation and balancing, followed by model training using diverse AI models and 5-fold cross-validation. The models were compared using ROC curves to select the most accurate model. Model interpretability was achieved through SHAP plots, aiding in understanding the feature contributions in the best-performing model.
Discussion
In this study, we applied an XAI model for long-term all-cause mortality in patients with PD using administrative healthcare data. Overall, our results showed that the XGBoost model had the best performance for predicting both 10- and 5-year mortality with AUROC values of 0.836 and 0.894. The most important contributing feature to PD mortality was age, followed by male sex and pneumonia. With regard to comorbidities, brain diseases such as dementia and stroke were associated with increased mortality, whereas dyslipidemia was inversely associated with mortality in patients with PD. Anthropometric data, such as BMI, Hb, glucose, and blood pressure, were also presented as important features for mortality prediction, showing a closer association with 5-year than 10-year mortality in patients with PD. We further demonstrated a nonlinear association between continuous variables and PD mortality using the XAI model. In addition, we demonstrated the feasibility of the prediction model for individualized 10-year mortality risk for each participant with PD using preexisting health care data.
PD is the fastest-growing neurological disease worldwide17. An increased mortality risk in PD has been suggested in many previous studies; however, risk factors for PD mortality have been investigated relatively scarce. PD-specific factors have been focused, and motor symptoms and cognitive impairment were suggested as risk factors for PD mortality7,18. When predicting mortality in PD, other medical conditions may be important contributing factors, considering that PD primarily occurs in older adults. Therefore, in this study, we used claims data to investigate various sociodemographic factors and combined diseases to predict all-cause mortality in patients with PD. Without clinical data for each participant with PD, we constructed a mortality prediction model with a high AUROC of 0.836 for the 10-year mortality and an increased AUROC of 0.894 for the 5-year mortality. The PD type and motor symptoms have been significantly associated with PD progression in many previous studies. Without these clinical symptoms, we constructed a highly accurate PD mortality prediction model, which suggested that the general condition of PD participants, including comorbid diseases, played an important role in mortality prediction of individuals of PD. Inputting clinical data of PD participants could further increase the performance of an AI model and is recommended for future studies.
In this study, XAI enabled us to identify a variety risk factors and complex associations between these factors and mortality in patients with PD. With regard to sociodemographic factors, age and male sex were identified as the most important factors in mortality prediction in patients with PD, which is in line with previous studies1,6,18. Interestingly, in PD patients < 70 years, age was the most important predictive factor, whereas male sex contributed the most to increased mortality in PD patients ≥ 70 years. Socioeconomic factors have been relatively less investigated for PD mortality in previous studies9,19. In this study, we used residential area, income level, and insurance type as input data, but these were not highly ranked as contributing factors to PD mortality. In our previous study investigating the association between socioeconomic status and mortality of PD in Korean populations, deprived socioeconomic status, such as income level and insurance type, showed some associations with increased mortality in PD9. In Korea, PD is registered as a rare and intractable disease, and patients receive additional medical expense support from the government. Overall, these results suggest that socioeconomic factors appear to have less influence on PD mortality than medical conditions in the Korean population. To confirm our results, the association should be validated in other ethnic groups with different healthcare systems.
In our XAI-based PD mortality prediction model, comorbidities were highly ranked as contributing factors. Pneumonia was the third most important risk factor for mortality, followed by age and sex, in both the 5- and 10-year XAI models. Considering that pneumonia is the most important cause of death in PD20,21, our XAI model appears to be accurate and feasible for clinical applications. Brain diseases including dementia, stroke, and traumatic brain injury, were highly ranked in the prediction model, particularly for 10-year mortality. Dementia and cognitive impairment have been shown to be associated with increased mortality in patients with PD1,6. Stroke and TBI have shown a positive association with PD22,23, but their impact on PD mortality has rarely been investigated. Our XAI models showed a positive association between brain diseases and PD mortality, which raises the need to pay attention to PD with combined brain disease for long-term management. Dyslipidemia was negatively associated with mortality in the prediction model. Previous studies have identified a complex association of PD with dyslipidemia, cholesterol level, and statin use, with these relationships still under investigation24,25. In this study, we included cholesterol level as the input data; however, it was not ranked among the top 20 contributing factors. Based on this, statin use for dyslipidemia, but not cholesterol level, appeared to influence mortality risk; however, the exact pathomechanism needs to be investigated in future studies. Interestingly, gastroesophageal reflux disease, dyspepsia, shoulder disease, and bronchitis were among the top 20 factors in the prediction model which were negatively associated with PD mortality. These comorbidities are prevalent and usually result in mild symptoms that increase hospital utilization. A possible reason is that these diseases were not actual contributors to PD mortality, but rather reflected real healthcare utilization in claims data. In other aspects, these comorbidities may have been overlooked as protective factors against PD mortality. The causality between the input data and outcomes cannot be driven by AI models, and future studies are required to unveil these associations.
The factors contributing to PD mortality differed between the 5-year and 10-year mortality prediction models. Overall, anthropometric data and laboratory values ranked highly in the 5-year mortality prediction model. In this study, data collected within two years of PD onset were used. Because anthropometric and laboratory data can change over time, the baseline data were more accurately reflected and showed increased prediction performance in the 5-year prediction model. In Korea, the national health screening program (NHSP) is implemented biannually by the government, and the inclusion of follow-up data and consideration of changes in these data could improve the prediction performance in a 10-year prediction model. We investigated the complex associations between contributing factors and PD mortality and elucidated the non-linear association of these variables with PD mortality.
Using the SHAP dependence plot, we identified nonlinear associations between key clinical factors and PD mortality risk, which enhances the clinical interpretability of our model. For example, BMI exhibited an inverse association with mortality risk up to ~25, beyond which the reduction in mortality plateaued. Similarly, hemoglobin levels around 15 mg/dL and fasting glucose levels near 100 mg/dL corresponded to the lowest predicted mortality risk. These findings suggest potential physiological ranges that may serve as optimal targets for risk stratification and patient management. However, it is important to note that while these values were derived from our model’s predictive patterns, they should not be interpreted as definitive clinical intervention thresholds. Further validation through prospective and interventional studies is required to confirm their applicability in clinical settings.
Individualized mortality risk prediction was possible at the time of PD diagnosis in our XAI model, which enabled risk stratification for mortality in individuals with PD. Despite of non-modifiable risk factors such as age and sex, modifiable factors such as BMI and laboratory values can be managed to reduce mortality. In addition, our model suggested optimal target values predicting lowest mortality for some variables. Identifying individual risk and protective factors would be helpful in disease management in real word practice, thereby increasing clinical applicability. For example, a PD patient with multiple risk factors, such as old age, male sex, and AD, should manage weight and laboratory values along with greater efforts to offset risk factors for mortality.
To our knowledge, this is the first attempt at applying an XAI prediction model for long-term mortality prediction in individuals with PD using nationwide population-based cohort data. This study had several implications. Large amounts of data are required for the development of AI prediction models, which requires considerable time and effort. In this study, we used preexisting healthcare data, the Korean NHIS data, that encompassed various sociodemographic factors and medical facility utilization. Although the clinical symptoms of PD were not included as input data owing to the nature of the claims data, we constructed a highly accurate mortality prediction model. Second, individualized mortality risk prediction was available at the time of PD diagnosis using only the claims data in our XAI model. Considering each person’s characteristics and the complex associations between them, an individualized prediction of long-term mortality could be made. The explainability of the XAI can suggest risk and protective factors for mortality prediction at the individual level, which can enable patient-centered applications to improve modifiable risk factors. Third, we elucidated the nonlinear association of various factors with the mortality risk in PD, further suggesting optimal target values for reducing mortality. Finally, using XAI models, less-appreciated factors that could be associated with PD mortality were identified. Whether these factors are associated with PD mortality should be investigated in future studies to confirm the associations.
Our study has some limitations. First, this study used claims-based cohort data; therefore, clinical symptoms such as motor symptoms and cognitive impairment were not included. The inclusion of clinical features in addition to claims data may improve the prediction performance of mortality risk and should be considered in future studies. Second, this study did not consider variables that could change over time. Given the high prediction performance of 5-year mortality with baseline data at PD diagnosis, consideration of changes in anthropometric or laboratory data can improve 10-year mortality prediction. Third, the AI model was applied and validated only in the Korean population. Ethnic differences in PD and healthcare policies across countries may result in different predictive outcomes in different regions. Validation with patients of other ethnicities is therefore required to generalize our results.
Methods
Data source
The National Claims Database of the NHIS of Korea was used for this study. This database encompasses the medical records of the entire Korean population covered by the mandatory NHIS and Medical Aid programs. We included sociodemographic information and a healthcare utilization data, for both inpatient and outpatient visits, encompassing diagnoses, procedures, surgeries, and prescription records. Diagnoses were made in accordance with the nomenclature of the International Classification of Disease-10th Revision-Clinical Modification (ICD-10-CM). The NHIS not only provides claims data, but also health screening examination data, offering a free biannual NHSP to all beneficiaries aged 20 years and above. The database contains patient history, physical examinations, anthropometric measurements, and questionnaire data, including lifestyle factors. This study was approved by the Institutional Review Board of Yonsei University, which waived the requirement for informed consent.
Study population and outcome
In 2006, the Korean government introduced a registration program for rare intractable diseases to assist patients with medical expenses by assigning disease-specific codes. The rare intractable disease code for PD is “V124”. In this study, we screened all newly diagnosed PD patients, based on allocation of the ICD-10 code G20 and rare intractable disease registration code V124, between January 2008 and December 2017. We excluded those diagnosed with secondary or atypical parkinsonism (ICD-10 codes G21-G23). Individuals diagnosed with PD before 2009 were also excluded; as such, we included only new patients diagnosed with PD after 2009 (washout period, 2008). Subsequently, we identified individuals who had participated in the NHSP at least once within 2 years of their initial diagnosis. Individuals with missing data were excluded. Finally, we longitudinally followed 36,480 individuals with PD to predict 10-year and 5-year mortalities. The endpoint of this study was all-cause mortality in patients with PD until December 31, 2020. Pipeline for XAI-based all-cause mortality prediction in PD is displayed in Fig. 5.
ROC curves showing the performance of seven different AI models in predicting 10-year mortality after PD. Each curve represents the trade-off between the true positive rate (sensitivity) and false positive rate (1-specificity), with the AUROC quantifying the discriminative ability of the model. The XGBoost (red line) achieved the highest AUROC of 0.894, followed closely by Gradient Boosting (blue line) at 0.890, and Random Forest (purple line) at 0.882. The Neural Network (pink line) demonstrated an AUROC of 0.834, whereas Logistic Regression (green line) yielded an AUROC of 0.833. The decision Tree (brown line) and naïve Bayes (yellow line) models exhibit lower AUROC s of 0.716 and 0.735, respectively. The diagonal gray line represents a no-skill classifier with an AUROC of 0.5.
Data extraction and preprocessing
We used the following 165 input variables: five sociodemographic features including age, sex, income levels, residential area, and insurance type from the participant insurance eligibility database; ten features including self-reported questionnaires about lifestyle factors, health profiles, and laboratory findings from the health screening database; and 150 features of the most prevalent comorbid diseases based on ICD-10 codes (see Supplementary Table 1 for detailed information).
Demographic variables such as age and sex were based on data entered at the time of PD diagnosis. Residential areas were categorized into urban and rural. The NHI premium is used as a proxy measure of income as it is proportional to monthly income, including earnings and capital gains. The income deciles of the enrolled individuals were categorized into four groups (Q1, Q2, Q3, and Q4, indicating all medical aid enrollees + 0–20, 21–50, 51–80, and 81–100 percentiles of NHI enrollees, respectively). Insurance type was categorized as either “National health insurance” or “Medical aid”. All NHSP participants were required to complete the self-report questionnaires on their health lifestyles. Lifestyle factors were categorized as follows; Smoking habits (non-smoker, ex-smoker, current smoker), weekly drinking consumption (0 times, 1 time, 2–3 times, 4–5 times, 6 or more times), and physical activity (inactive, moderate, vigorous). Anthropometric data, including BMI, waist circumference, and blood pressure (systolic and diastolic), were assessed. Venous samples were drawn after overnight fasting to determine total cholesterol, Hb, and fasting glucose levels. Comorbidities developing within 2 years of PD diagnosis were investigated, and the top 150 most frequent comorbidities were included in the prediction model.
For model training and validation, the entire dataset was divided into the training and test sets in a ratio of 7:3. The training set was randomly partitioned into five subsets of almost equal size for five-fold cross-validation26. The dataset for the 10-year mortality prediction was balanced; however, the dataset for 5-year mortality prediction was imbalanced; the number of participants who died within 5 years of PD diagnosis (19%) was much lower than that of those who survived (81%). Therefore, we performed bootstrap sampling with 10 replacements to obtain a balanced dataset during cross-validation and improve the prediction accuracy.
Model implementation and evaluation
Seven ML algorithms were implemented: logistic regression, random forest, neural network, Gradient Boosting Machines (GBM), decision tree, naïve Bayes, and extreme Gradient Boost (XGBoost). Each model was optimized through hyperparameter tuning, a process of systematically adjusting algorithm parameters to enhance predictive accuracy. The optimized hyperparameters for each ML model are detailed in Supplementary Table 4. Model performance metrics were measured with the test dataset based on several evaluation metrics, including AUROC, accuracy, sensitivity, specificity, recall, and MCC27,28,29. Confidence intervals (CI) for AUROC were derived through resampling using the bootstrap percentile method with 1000 repetitions30. The ML algorithms used in our study were applied using R version 3.3.3, including the packages caret31, Neural Network32, Random Forest33, e107134, rpart35, GBM36, XGBoost37, ROCR38, and pROC39. We built three prediction models to determine how the predictive performance of ML algorithms changed according to various factors, including demographic and anthropometric factors, socioeconomic status, and comorbidities.
Model interpretation
We used the SHAP value to identify the principal features driving model prediction. This index is based on the principles of cooperative game theory and the importance value obtained by probabilistically calculating the contribution of players (features) in the game to the final game outcome (mortality) using the Shapley value40. By averaging across samples, SHAP values estimate the contribution of each feature to overall model predictions41. Here, higher SHAP values indicate larger contributions to mortality risk. These values are suitable for addressing the trade-off between interpretability and accuracy in complex models with excellent accuracy, such as XGBoost, artificial neural networks, and gradient-boosting machines42,43.
To interpret our ML models, SHAP analysis was applied to the ML prediction model that demonstrated the highest predictive performance. The SHAP values were extracted separately for 10-year and 5-year mortality prediction models. In addition, considering that age is an important prognostic factor for PD, SHAP values stratified by age of 70 years were also extracted.
Statistical analyses
The baseline characteristics of the patients with PD who died and those who did not die were compared using Student’s t-test for continuous variables and the chi-square test for categorical variables. All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA), with the statistical significance level set at P < 0.05.
Data availability
This study was performed using data extracted from the NHIS database (https://nhiss.nhis.or.kr/); however, restrictions apply to the availability of these data, which were used under license for the current study and are therefore not publicly available. The source NHIS data do not belong to the researchers, and we are not allowed to transfer the data files to a third party under Korean law. The data were used after obtaining approval from the Institutional Review Board and Korea NHIS Big Data Operations Department.
Code availability
The underlying code used in this study was tailored to the NHIS data and structure. Therefore, the code has little use without access to data and, as such, has not been made publicly available. The code for this study may be made available to qualified researchers upon reasonable request from the corresponding author. All data processing and modeling were conducted in R version 3.3.3 using publicly available standard libraries, which are described in the Methods section.
References
Macleod, A. D., Taylor, K. S. & Counsell, C. E. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 29, 1615–1622 (2014).
Chaudhuri, K. R., Healy, D. G. & Schapira, A. H. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 5, 235–245 (2006).
Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov. Disord. 17, 60-67, (2002).
Yoon, S. Y., Shin, J., Kim, Y. W., Chang, J. S. & Won Kim, H. The mortality rate of Parkinson’s disease and related comorbidities: a nationwide population-based matched cohort study in Korea. Age Ageing 50, 1182–1188 (2021).
Bäckström, D. et al. Early predictors of mortality in Parkinsonism and Parkinson disease: a population-based study. Neurology 91, e2045–e2056 (2018).
Hoogland, J., Post, B. & de Bie, R. M. A. Overall and disease related mortality in Parkinson’s disease—a longitudinal cohort study. J. Parkinsons Dis. 9, 767–774 (2019).
Marras, C. et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology 64, 87–93 (2005).
Pohar, S. L. & Allyson Jones, C. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch. Gerontol. Geriatr. 49, 317–321 (2009).
Yoon, S. Y., Shin, J., Chang, J. S., Lee, S. C. & Kim, Y. W. Effects of socioeconomic status on mortality after Parkinson’s disease: a nationwide population-based matched cohort study in Korean populations. Parkinsonism Relat. Disord. 80, 206–211 (2020).
Searles Nielsen, S. et al. A predictive model to identify Parkinson disease from administrative claims data. Neurology 89, 1448–1456 (2017).
Lee, W. H. et al. Development of a novel prognostic model to predict 6-month swallowing recovery after ischemic stroke. Stroke 51, 440–448 (2020).
Feng, Z. et al. Machine learning based on clinical characteristics and chest CT quantitative measurements for prediction of adverse clinical outcomes in hospitalized patients with COVID-19. Eur. Radio. 31, 7925–7935 (2021).
Angraal, S. et al. Machine learning prediction of mortality and hospitalization in heart failure with preserved ejection fraction. JACC Heart Fail 8, 12–21 (2020).
Savage, N. Breaking into the black box of artificial intelligence. Nature https://doi.org/10.1038/d41586-022-00858-1 (2022).
Joyce, D. W., Kormilitzin, A., Smith, K. A. & Cipriani, A. Explainable artificial intelligence for mental health through transparency and interpretability for understandability. npj Digit. Med. 6, 6 (2023).
Garriga, R. et al. Machine learning model to predict mental health crises from electronic health records. Nat. Med. 28, 1240–1248 (2022).
Dorsey, E. R., Sherer, T., Okun, M. S. & Bloem, B. R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 8, S3–s8 (2018).
Zhang, Y. et al. Mortality from Parkinson’s disease in China: findings from a ten-year follow up study in Shanghai. Parkinsonism Relat. Disord. 55, 75–80 (2018).
Yang, F. et al. Socioeconomic status in relation to Parkinson’s disease risk and mortality: a population-based prospective study. Medicine 95, e4337 (2016).
Pennington, S., Snell, K., Lee, M. & Walker, R. The cause of death in idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 16, 434–437 (2010).
Fall, P. A., Saleh, A., Fredrickson, M., Olsson, J. E. & Granérus, A. K. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: a 9-year follow-up. Mov. Disord. 18, 1312–1316 (2003).
Ascherio, A. & Schwarzschild, M. A. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272 (2016).
Liu, Y., Xue, L., Zhang, Y. & Xie, A. Association between stroke and Parkinson’s disease: a meta-analysis. J. Mol. Neurosci. 70, 1169–1176 (2020).
Huang, X. et al. Statins, plasma cholesterol, and risk of Parkinson’s disease: a prospective study. Mov. Disord. 30, 552–559 (2015).
Rozani, V. et al. Higher serum cholesterol and decreased Parkinson’s disease risk: a statin-free cohort study. Mov. Disord. 33, 1298–1305 (2018).
Cawley, G. C. & Talbot, N. L. On over-fitting in model selection and subsequent selection bias in performance evaluation. J. Mach. Learn. Res. 11, 2079–2107 (2010).
Chicco, D. & Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21, 1–13 (2020).
Halimu, C., Kasem, A. & Newaz, S. S. In Proceedings of the 3rd international conference on machine learning and soft computing. 1–6 (ICMLSC, 2019).
Jiao, Y. & Du, P. Performance measures in evaluating machine learning based bioinformatics predictors for classifications. Quant. Biol. 4, 320–330 (2016).
Cortes, C. & Mohri, M. Confidence intervals for the area under the ROC curve. Advances in neural information processing systems, vol. 17 (2004).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26 (2008).
Ripley, B., Venables, W. & Ripley, M. B. Package ‘nnet’. R package version 7 700 (2016).
Liaw, A. & Wiener, M. Classification and regression by randomForest. R. N. 2, 18–22 (2002).
Dimitriadou, E., Hornik, K., Leisch, F., Meyer, D. & Weingessel, A. Misc functions of the department of Statistics (e1071). TU Wien. R. package 1, 5–24 (2008).
Therneau, T., Atkinson, B. & Ripley, B. rpart: recursive partitioning and regression trees. R package version vol. 4, 1–9 (2015).
Ridgeway, G. Generalized boosted models: a guide to the gbm package. Update 1 (2007).
Chen, T., He, T., Benesty, M. & Khotilovich, V. Xgboost: extreme gradient boosting. R package version 0.4-2, 1-4 (2015).
Sing, T., Sander, O., Beerenwinkel, N. & Lengauer, T. ROCR: visualizing classifier performance in R. Bioinformatics 21, 3940–3941 (2005).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma. 12, 77 (2011).
Lundberg, S. M. & Lee, S.-I. A unified approach to interpreting model predictions. Advances in neural information processing systems, vol. 30 (2017).
Merrick, L. & Taly, A. In Machine Learning and Knowledge Extraction: 4th IFIP TC 5, TC 12, WG 8.4, WG 8.9, WG 12.9 International Cross-Domain Conference, CD-MAKE 2020, Proceedings 4. 17-38 (Springer, 2020).
Lundberg, S. M. et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat. Biomed. Eng. 2, 749–760 (2018).
Vilone, G. & Longo, L. Notions of explainability and evaluation approaches for explainable artificial intelligence. Inf. Fusion 76, 89–106 (2021).
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2024-00337352).
Author information
Authors and Affiliations
Contributions
Y.H.P. conceptualized the study, performed the data analysis, wrote the main manuscript text, and prepared figures 1–5. Y.W.K. and S.C.L. contributed to the study design, clinical data interpretation, and manuscript revisions. D.R.K. provided statistical oversight and contributed to data analysis and interpretation. S.Y.Y. supervised the overall project, contributed to the study design, and critically revised the manuscript for important intellectual content. S.Y.Y. is the corresponding author. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, Y.H., Kim, Y.W., Kang, D.R. et al. Prediction of all-cause mortality in Parkinson’s disease with explainable artificial intelligence using administrative healthcare data. npj Parkinsons Dis. 11, 144 (2025). https://doi.org/10.1038/s41531-025-01007-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01007-x