Abstract
Parkinson’s disease (PD) is a neurodegenerative disease characterized by motor and non-motor symptoms that progressively deteriorate and for which there is no disease-modifying pharmacological treatment. Exercise is widely recommended for individuals with PD due to its potential neuroprotective benefits. However, the mechanisms underlying these exercise-induced effects in PD remain poorly understood. Analyzing fluid biomarkers responsive to exercise could offer valuable insights into the mechanisms by which exercise impacts PD and aid in optimizing exercise prescriptions for individuals with PD. This review explores exercise-responsive biomarkers categorized into three key groups—neurotrophic, inflammatory, and neuroendocrine markers. It highlights both well-validated biomarkers and candidates with promising potential. We also highlight key biomarkers linked to PD pathology, such as α-synuclein, and their potential connection to exercise based on current evidence. Comprehensive characterization of these biomarkers will advance our understanding of the biological effects of exercise in PD, enabling mechanism-based and objective measures to evaluate exercise response in future clinical trials and its impact on PD signs and symptoms.
Similar content being viewed by others
Introduction
The prevalence of PD has doubled over the past 25 years and is projected to exceed 12 million cases by 20401,2. This rapid increase is driven by an aging population with longer lifespans and environmental factors such as pollution. PD is an extremely complex disorder with numerous etiological factors, diverse pathogenic pathways, and a myriad of motor and non-motor symptom manifestations that vary in progression between individuals3. Strategies targeting specific pathogenic pathways have thus far been unsuccessful in modifying disease progression4,5. In this review, we explore a therapy with broad therapeutic potential – aerobic exercise – and discuss how it may influence multiple pathogenic pathways simultaneously. Specifically, we discuss the changes in circulating biomarkers in the context of PD and in response to exercise to provide insights into how exercise can impact brain health and PD pathogenesis.
Complexity of PD
One of the major challenges in PD is its heterogeneity, with signs and symptoms encompassing motor, cognitive, behavioral, autonomic, and sleep disturbances, as well as variability in the rate of disease progression6. Although the majority of cases of PD appear to be sporadic, evidence supports predisposing factors including environmental toxic exposure and genetic defects7. The hallmark motor symptoms primarily stem from the progressive loss of nigrostriatal dopaminergic neurons. However, other brain regions are significantly affected and are responsible for treatment-resistant motor and non-motor symptoms, which often manifest decades before the onset of motor signs8. Neurodegeneration in PD may be a result of diverse pathogenic pathways, including the toxicity of soluble and insoluble aggregated α-synuclein, mitochondrial dysfunction, defective proteolysis due to ubiquitin-proteasome system dysfunction, oxidative stress, and inflammation9,10,11.
Current strategies to modify disease progression in PD have included agents targeting α-synuclein accumulation or cell-to-cell transmission, specific organelles such as mitochondria and lysosomes, associated proteins like β-glucocerebrosidase and leucine-rich repeat kinase 2, neuronal rescue pathways such as calcium channel blockers and iron reducers, and molecules involved in neuroinflammatory pathways4. Despite these efforts, no pharmacological treatment has proven effective in modifying disease progression.
Exercise and Clinical Symptoms of PD
Multiple clinical studies and meta-analyses highlight the benefits of various exercise modalities on improving motor symptoms, muscle strength, functional mobility and balance, and quality of life in individuals with PD12,13. Most studies have focused on three main interventions: aerobic exercise, resistance exercise, and balance exercises. Additional studies have investigated the effects of mind-body practices such as Tai Chi, Yoga, and Health Qigong14,15. Parameters such as the intensity (low, moderate, or high), frequency, muscle contraction types (e.g., eccentric vs. concentric), interval vs. continuous training, and the setting (home vs. supervised) vary by exercise modality and study design. It is beyond the scope of this review to cover all exercise types, so we direct readers to published reviews on other exercise modalities and their effects on PD symptoms13,16,17,18. Furthermore, practical guidelines for exercise prescriptions are available19.
The present review focuses on long-term (chronic) aerobic exercise training, as it is the form of exercise that has the most compelling evidence for slowing disease progression and reducing symptoms in PD, based on extensive preclinical studies and clinical studies20,21,22,23. The primary goal of aerobic exercise training is to enhance the capacity of the circulatory and respiratory systems to supply oxygen to skeletal muscles and to sustain prolonged, rhythmic activity. Activities such as cycling, running, rowing, brisk walking, and swimming achieve this by increasing breathing and heart rates.
Longitudinal observational cohort studies have demonstrated that individuals with PD who exercise regularly experience a slower progression of motor and cognitive symptoms and report better quality of life outcomes compared to non-exercisers24,25,26. Furthermore, strong evidence suggests that exercise reduces the risk of developing clinical PD27,28, potentially by influencing fundamental pathogenic processes. To date, three randomized controlled trials have shown that moderate (50-70% of maximum heart rate or heart rate reserve) to high-intensity (80–85% of maximum heart rate or heart rate reserve) aerobic exercise has the potential to slow disease progression and reduce the signs and symptoms of PD20,21,22. Data from these trials also suggest a dose-response relationship between aerobic exercise intensity and motor function improvements in PD, with higher aerobic exercise dosage (number of sessions x session duration) yielding greater effects on motor symptoms. However, evidence is needed to confirm this relationship.
A recent study by De Laat et al. demonstrated that 6 months of high-intensity exercise in patients with mild and early PD (n = 10) reversed the expected decrease in dopamine transporter availability on 18F-FE-PE2I PET imaging, showing a significant increase in both the substantia nigra and putamen29. Additionally, exercise reversed the expected decline in neuromelanin concentration in the substantia nigra, resulting in a significant increase. These findings suggest that exercise may enhance the dopaminergic system and deserves further investigation.
Studies examining the effect of aerobic exercise on cognition in PD have yielded conflicting results. While some studies reported improvement in cognitive function30,31, others showed no significant changes21,32. Similarly, there is insufficient evidence to support a beneficial effect of aerobic exercise training on non-motor symptoms of PD, including depression, apathy, sleep disturbances, fatigue, or constipation33. Exercise, however, has the potential to improve dysregulated crosstalk between the brain and peripheral systems and to improve both motor and non-motor symptoms, many of which are thought to originate in the periphery34,35,36. Assessing biomarker changes in conjunction with symptom improvement will provide stronger mechanistic evidence for the role of exercise in PD.
A Role for Biomarkers in Understanding the Effects of Exercise
Given the complexity surrounding biomarker definitions and their application in research and clinical practice, the NIH and FDA have created the “Biomarkers, Endpoints, and other Tools” (BEST) resource. The BEST resource categorizes biomarkers into seven types along the clinical continuum of disease: risk/susceptibility, diagnostic, predictive, prognostic, monitoring, safety, and response37. Among these, response biomarkers indicate a biological response to a therapy or environmental agent. This review focuses on response biomarkers that may reflect the diverse mechanisms by which exercise benefits individuals with PD. Some biomarkers may have application in different categories. There have been promising developments in detecting abnormal α-synuclein for diagnosis of PD with Lewy bodies as described in a later section. However, the reliability of other biomarker categories, such as risk/susceptibility, predictive/prognostic and monitoring biomarkers, remains under investigation in PD and will not be discussed here.
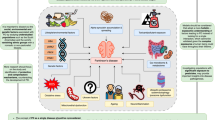
Exercise has demonstrated neuroprotective effects in animal models of PD23,38,39,40. These effects are thought to result from neurogenesis, synaptogenesis, and angiogenesis and from modulation of mitochondrial function, oxidative stress, and neuroinflammation41,42,43. Identifying exercise-responsive biomarkers that influence these potentially disease-modifying mechanisms is crucial. The ongoing SPARX3 (Study in Parkinson’s Disease of Exercise Phase 3 Clinical Trial) aims to identify key biomarkers that respond to moderate- and high-intensity aerobic exercise in people with PD44. Figure 1 presents an overview of mechanisms involved in the beneficial effects of chronic aerobic exercise.
Chronic aerobic exercise leads to liberation of neurotrophic factors, modulation of inflammatory factors, and modulation of neuroendocrine factors. Downstream mechanisms may include enhanced neuronal synthesis, structural and behavior adaptations. Adapted from Paillard et al. 2023. BDNF brain-derived neurotrophic factor, GDNF glial cell line-derived neurotrophic factor, IGF-1 insulin-like growth factor 1, VEGF vascular endothelial growth factor, GPLD1, glycosylphosphatidylinositol-specific phospholipase D1, TNF tumor necrosis factor, IL-6 interleukin-6, CRP C-reactive protein.
Exercise-Induced Changes in Neurotrophic/Neuroprotective Markers
While the pathophysiological mechanisms underlying PD remain unclear, it is well-established that neurological symptoms are a consequence of dopaminergic neuronal loss45. Extensive research has focused on the role of neurotrophic factors as protective agents against neuronal damage in PD46,47. Neurotrophic factors are secreted peptides that play key roles in development, proliferation, protection, survival, and restoration of neuronal cells. These factors have shown potential for disease modification in preclinical PD models, and clinical studies have linked changes in neurotrophic factors levels to motor and non-motor symptoms of PD46,47,48,49,50.
In individuals with PD, alterations in neurotrophic factors have been reported in post-mortem brains, cerebral spinal fluid (CSF), and blood. Specifically, brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), and glial cell line-derived neurotrophic factor (GDNF) levels have been reported to be decreased in post-mortem PD brains51,52,53,54,55 and CSF56, as well as in peripheral blood57,58,59,60. Conversely, cerebral dopamine neurotrophic factor (CDNF) and insulin-like growth factor 1 (IGF-1) levels are elevated in post-mortem brains and CSF, likely as part of a compensatory protective mechanism54,61. Similarly, serum levels of IGF-1 and mesencephalic astrocyte-derived neurotrophic factor (MANF) are increased in PD61,62. Together, this evidence suggests that both peripheral and central neurotrophic factor levels are dysregulated in PD.
Neurotrophic factors represent a leading category of biomarkers that mediate many of the benefits of exercise. In animal models, exercise robustly increases levels of BDNF, GDNF, VEGF, IGF-1, NGF, and CDNF41,50,63,64. The effects depend on exercise type, intensity, and whether changes are acute or chronic. In this review, we focus on BDNF, GDNF, VEGF and IGF-1 as principal neurotrophic factors to assess responses to chronic exercise because their levels are altered in PD51,52,53,54,55,56,57,58,59,60,61,62. Their circulatory levels consistently increase with long-term exercise, and these growth factors exert complementary effects, supporting neuronal survival and regulating brain plasticity and function (Fig. 2). Additionally, we discuss other biomarkers, including irisin, glycosylphosphatidylinositol-specific phospholipase D1 (GPLD1), sirtuin-3 (SIRT3), and lactate. Emerging evidence suggests these biomarkers respond to chronic exercise, potentially mediating peripheral tissue-brain crosstalk, and they have neuroprotective effects.
Chronic aerobic exercise stimulates elevations in multiple neurotrophins, with a most robust response in BDNF, VEGF, and IGF-1 in the periphery and in the CNS. Irisin, GPLD1, SIRT3, and lactate are involved in tissue-brain crosstalk to mediate positive benefits of exercise, with one mechanism being an increase in neurotrophins such as BDNF by GPLD1. Elevation in neurotrophic factors has been linked to pathways that counter neurodegeneration and synaptic plasticity. BDNF brain-derived neurotrophic factor, GDNF glial cell line-derived neurotrophic factor, IGF-1 insulin-like growth factor 1, VEGF vascular endothelial growth factor, GPLD1 glycosylphosphatidylinositol-specific phospholipase D, SIRT3 sirtuin-3. Created in BioRender. Mehta, N. (2025) https://BioRender.com/a05k823.
BDNF
BDNF plays a key role in neuronal maturation and survival, adult hippocampal neurogenesis, and neural plasticity65. Experimentally, inhibition of BDNF mRNA expression via antisense oligonucleotide infusions results in the loss of nigral dopaminergic neurons66, providing evidence that reduced BDNF may contribute significantly to the degeneration of dopaminergic neurons in PD. Conversely, boosting BDNF expression through gene therapy in PD rat models has been demonstrated to prevent the loss of nerve terminals and cell bodies of the nigrostriatal dopaminergic pathway, while also inducing sprouting of dopaminergic fibers67,68. In clinically and neuropathologically typical PD, BDNF mRNA expression is reduced by 70% in the substantia nigra pars compacta (SNpc), attributed to both the loss of dopaminergic neurons that produce BDNF and reduced BDNF mRNA expression in surviving dopaminergic neurons69. Decreased levels in BDNF have also been reported in the blood of individuals with PD57,60. Moreover, BDNF levels have been found to negatively correlate with age at PD onset and with non-motor symptoms such as impaired cognition and depression70,71. Interestingly, serum BDNF levels positively correlate with a longer duration of disease, increased symptom severity, and more advanced stage of PD72. One possible explanation is that lower BDNF levels may contribute to the initial risk and pathogenesis of PD risk, while increased levels during disease progression could represent a compensatory mechanism. This dual role - both a reduction and a compensatory increase in BDNF - suggests that the effects of exercise on BDNF may depend on the stage of the disease.
It is well established that exercise increases BDNF in skeletal muscle, promoting paracrine effects that influence motor neuron innervation73. Voluntary exercise in mice increases BDNF levels in the dorsal striatum and stimulates dopamine release74. BDNF is also necessary for exercise-induced increases in hippocampal dentate gyrus, which enhances memory and learning75,76. In animal models of PD, alleviation of motor deficits following exercise is directly linked to increased BDNF levels39,77. There is robust evidence of an increase in blood BDNF levels with chronic aerobic exercise in healthy adults78,79,80. A recent systematic review by Paterno et al. analyzed 16 studies (8 two-arm trials and 8 single-arm trials) involving 370 PD participants and found significant rises in serum or plasma BDNF levels after 4–12 weeks of chronic aerobic or multimodal exercise, including aerobic training81. Both exercise intensity and total exercise volume were positively associated with BDNF changes. Similarly, Kaagman et al. conducted a meta-analysis of 5 trials (n = 216 participants with PD), revealing significant changes in serum or plasma BDNF levels with chronic aerobic training82. Furthermore, BDNF changes correlated with improvements in motor performance, measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) part III motor score, as well as ambulatory capacity and balance82. These findings suggest that BDNF may play a key role in mediating the motor benefits of exercise in PD.
GDNF
GDNF is expressed in the striatum and other regions that receive dopaminergic input from SNpc and motor neurons. Two studies have reported lower serum GDNF levels in individuals with PD compared to healthy controls, with this reduction associated with cognitive impairment59,83. Additionally, post-mortem analyses have shown reduced GDNF levels in the hippocampi of PD brains, despite no evidence of neuronal loss in that region54. Given its neuroprotective potential, GDNF administration has been explored as a therapeutic strategy in preclinical models of PD, where it has been shown to prevent nigral neuron loss and restore disease-associated neurochemical and behavioral changes84,85. Similarly, in Rhesus monkeys with toxin-induced parkinsonism, GDNF administration significantly improved postural instability, rigidity, and bradykinesia, while also enhancing dopamine levels and increasing dopaminergic fiber density86. Clinical trials investigating intraputaminal GDNF delivery in PD have reported marked improvements in UPDRS motor scores and “OFF” times in open-label studies87,88. However, subsequent double-blind, placebo-controlled trials did not show significant UPDRS motor score improvements and were discontinued due to excessive weight loss89,90,91. These findings suggest that while GDNF administration may offer benefits in PD, it is likely insufficient as a standalone treatment. Exercise, in contrast, may provide broader therapeutic effects, potentially including GDNF elevation as one of its mechanisms.
Chronic aerobic exercise has been shown to increase GDNF levels in the striatum, hippocampus, spinal cord, and muscle tissue in rodent models92,93,94,95,96. However, human studies on the effects of exercise on circulating GDNF levels remain limited. To date, only one prospective cohort study has demonstrated higher serum GDNF levels in healthy adults who engaged in aerobic exercise for six months compared to their sedentary counterparts97. Further research is needed to determine how circulating GDNF levels respond to aerobic exercise in individuals with PD.
IGF-1
Alongside BDNF and GDNF, IGF-1 plays a crucial role in mediating exercise-induced benefits on neuroplasticity. IGF-1 signaling regulates pathways that promote cell growth and survival, maturation, and proliferation, supporting tissue maintenance and repair98. Additionally, IGF-1 promotes angiogenesis and amyloid clearance99,100. IGF-1 has also been shown to enhance exercise-induced BDNF signaling, contributing to improvements in learning and memory101. Notably, blocking peripheral IGF-1 entry into the brain abolishes the neurogenic effects of exercise102.
In PD, multiple studies show that serum IGF-1 levels are elevated at diagnosis, possibly as a compensatory mechanism, but this increase diminishes as the disease progresses103,104,105,106. One study also reported elevated CSF IGF-1 levels at baseline in PD61. Lower serum IGF-1 levels at disease onset are associated with worse prognosis, increased cognitive impairment risk, and faster disease progression, suggesting that individuals who fail to exhibit an adequate IGF-1 increase experience poorer outcomes105,107. Several studies have examined the effects of chronic aerobic exercise on IGF-1 levels. Baker et al. found that a 24-week aerobic exercise program increased plasma IGF-1 levels in men with mild cognitive impairment (MCI), but not in women, and was associated with cognitive improvements108. In a meta-analysis, Nasir et al. reported a significant increase in IGF-1 in postmenopausal women following long-term aerobic exercise109. Other studies have reported either maintenance of IGF-1 levels following 7-24 weeks of aerobic exercise110,111,112 or a decrease after 48 weeks of moderate-intensity aerobic exercise113. Discrepancies in findings may stem from differences in comorbidities, intervention duration, and exercise intensity. Studies evaluating IGF-1 changes in response to exercise in PD remain limited. To date, only Stuckenschneider et al. have reported a slight improvement in serum IGF-1 levels following an 8-week multimodal exercise intervention (aerobic + resistance + balance/dual task) in individuals with PD114.
VEGF
VEGF plays a central role in brain angiogenesis, promoting neuronal survival and glial growth115. Like IGF-1, VEGF is essential for exercise-induced neurogenesis, as its peripheral blockade abolishes running-induced neurogenesis116. VEGF also exerts potent neuroprotective effects on dopaminergic neurons, likely through its angiogenic and glial-proliferative properties117. Notably, low-dose administration of VEGF-secreting cells provides significant neuroprotection when implanted into the striatum of PD rodent models118. Serum VEGF levels in individuals with PD have not shown to differ significantly from controls119. However, CSF levels of VEGF have been shown to be elevated in people with PD and PD dementia compared to healthy controls56. This increase is associated with greater gait difficulty, orthostatic hypotension, and increased blood-brain barrier (BBB) permeability. Higher CSF VEGF levels align with findings in postmortem PD brains, which exhibit increased angiogenesis120,121, and recent studies suggest that disruptions in these pathways contribute to disease progression56,122.
Experimental evidence indicates that exercise induces VEGF upregulation123,124. In rodent models, exercise enhances vascularization and VEGF levels in the substantia nigra, whereas aging and sedentary behavior are linked to reduced nigral microvascular density and VEGF mRNA expression125. A systematic review and meta-analysis by Song et al., which included 28 studies on chronic aerobic exercise in older adults, found that blood VEGF concentrations are higher post-exercise126. While no significant effect of exercise duration was observed, a subset of studies (n = 21) reported a trend toward increased VEGF levels following ≥4 weeks of aerobic exercise compared to a single exercise session126. These studies suggest that chronic exercise can enhance VEGF levels, potentially contributing to its neuroprotective effects in PD. However, more studies are needed to determine how VEGF changes with exercise in people with PD.
Irisin
Irisin is a myokine primarily expressed in skeletal muscle during exercise127. A secretory form of the transmembrane protein fibronectin type III domain containing 5, irisin has gained significant attention since its discovery in 2012 because of its role in regulating energy metabolism and its links to metabolic and neurodegenerative disorders128,129. It can cross the BBB and directly induce expression of hippocampal BDNF and other neuroprotective genes130,131. Additionally, irisin has been shown to protect against neuronal injury in ischemic conditions132,133. In preclinical models of PD, irisin administration rescues dopaminergic neurons from degeneration, restores mitochondrial biogenesis, and alleviates oxidative stress134,135. Kam et al. have shown that intravenous irisin delivery via viral vectors following intrastriatal injection of α-synuclein preformed fibrils in mouse models of PD reduces pathological α-synuclein accumulation, preserves dopamine neurons, prevents striatal dopamine depletion, and improves motor deficits136. In people with PD, Shi et al. reported that plasma irisin levels decline as the disease progresses, negatively correlating with plasma α-synuclein levels and positively correlating with dopamine uptake in the striatum137.
An increasing number of clinical trials are investigating how irisin responds to exercise in humans. Jandova et al. conducted a systematic review and meta-analysis on 51 studies examining chronic exercise in both healthy adults and individuals with conditions such as interstitial lung disease, progressive multiple sclerosis (MS), or type 2 diabetes138. Among these, 25 were randomized controlled trials. Within-group analyses showed that blood irisin levels increased in 23 studies and decreased in 10, with a statistically significant between-groups effect in 15 studies. Chronic aerobic training alone demonstrated a significant positive effect in 4 studies compared to non-exercise control groups138. The considerable heterogeneity in findings may stem from differences in training protocols, participant populations, and methodological aspects of irisin measurement138. One major challenge in clinical trials is the accuracy of circulating irisin measurements. The commonly used ELISA kit has been criticized for its low reliability due to variability in available antibodies. Albrecht et al.139 highlighted this issue, cautioning that ELISA-based results should be interpreted carefully until the reliability of irisin antibodies is validated. While mass spectrometry is more accurate, it requires complex, multi-step sample preparation that introduces additional variables.
Only one study thus far has evaluated the effects of chronic exercise on irisin levels in people with PD. Zhang et al. have shown that 12 weeks of regular aerobic exercise increased serum irisin levels, which positively correlates with improved balance function134.
GPLD1
GPLD1 is another exercise-induced circulating factor that may be crucial in the crosstalk between peripheral tissues and the brain during exercise140. As a hepatokine, GPLD1 has been implicated in neurogenesis and several neurodegenerative diseases141. In a novel approach, Horowitz at el. demonstrated that the transferring of plasma from exercised aged mice to sedentary aged mice conferred exercise-related benefits on adult neurogenesis and increased BDNF levels142. The study identified GPLD1 as a blood factor enriched in the transferred plasma and linked it to improved cognitive performance. Moreover, in vivo liver transfection to elevate GPLD1 levels led to increased BDNF expression in the hippocampus of aged mice142. Interestingly, GPLD1 does not readily cross the BBB, suggesting it mediates brain benefits indirectly through peripheral-brain signaling140. In humans, GPLD1 levels are higher in physically active elderly individuals (average daily steps ≥7100) compared to their sedentary counterparts (average daily steps <7100)142. However, no randomized controlled trials have yet examined the effects of exercise on GPLD1 in healthy adults or individuals with PD.
SIRT3
SIRT3 is gaining recognition as a potential neuroprotective factor in neurodegenerative diseases such as PD. As a key regulator of mitochondrial homeostasis, SIRT3 has been identified as a potential disease-modifying target, given that mitochondrial dysfunction is a hallmark of PD pathology143. By deacetylating mitochondrial enzymes, SIRT3 prevents age-related mitochondrial dysfunction and oxidative stress144. Additionally, it supports mitophagy to clear damaged mitochondria, induces autophagy in macrophages, and regulates NLRP3 inflammasome activation, contributing to neuroprotection and anti-inflammatory effects145,146.
Numerous studies have linked SIRT3 to longevity in humans and rodents147,148,149. Trinh et al. have reported that SIRT3 levels are reduced by over 50% in the SNpc of PD participants compared to healthy controls150. In rodent models of PD, SIRT3 overexpression prevents dopaminergic cell loss, decreases reactive oxygen species (ROS) production, and mitigates oxidative stress151,152,153. Oligomeric α-synuclein associates with mitochondria and reduces SIRT3 levels, whereas SIRT3 overexpression has been shown to reduce α-synuclein oligomer formation154.
Given its role in cellular aging, SIRT3 is increasingly studied in the context of exercise, a well-known anti-aging intervention155. Chronic aerobic exercise (≥8 weeks) increases SIRT3 in skeletal muscle and serum across all age groups156,157. Notably, SIRT3 expression is nearly 40% lower in sedentary older adults but remains elevated in aerobic-trained individuals, regardless of age158. Investigating SIRT3 response to aerobic exercise may provide further insight into its role as a key mediator of exercise-induced benefits in PD.
Lactate
Evidence over recent years has also pointed to lactate as an important mediator for the benefits of exercise on brain health159. Lactate was once considered a waste product responsible for muscle fatigue, but it is now recognized as a metabolic signaling molecule critical for brain function. It plays a crucial role in energy delivery, storage, production, and utilization160,161. Lactate exhibits dual roles: on one hand, it can be detrimental by increasing ROS production and oxidative stress; on the other hand, it is vital for maintaining brain homeostasis, promoting synaptic plasticity, neurogenesis, angiogenesis, and anti-inflammatory effects162. Liguori et al. have reported elevated CSF lactate levels in individuals with early PD, which correlated with clinical disease progression and neurodegeneration markers such as phosphorylated-tau163. Conversely, other studies have found no significant changes in serum lactate levels in individuals with moderately advanced PD164.
Chronic exercise induces metabolic adaptations, particularly in skeletal muscle, leading to reduced lactate production at a given intensity165. In people with PD, Martino et al. demonstrated that after 4 weeks of treadmill training, acute elevation in lactic acidemia is less pronounced166. Similar normalizations in lactate levels has been observed with chronic exercise in people with MS167 and type 2 diabetes168. However, further research is needed to confirm the specific effects of chronic exercise on lactate regulation in PD and its correlation with clinical symptoms.
Exercise-Induced Changes in Inflammatory Markers
Substantial evidence supports a pathogenic role of inflammation in PD. However, it remains unclear whether the initial source of inflammation originates in the central nervous system (CNS) or the periphery, as immune alterations are present in both compartments, even in early PD169. Notably, chronic inflammatory conditions such as inflammatory bowel disease (IBD), psoriasis, arthritis, and diabetes—each linked to increased PD risk—suggest that peripheral inflammation may act as a trigger for neuroinflammation in PD170.
In individuals with PD, certain inflammatory biomarkers are significantly altered in both the periphery and the CNS, while others show changes in only one compartment. A systematic review and meta-analysis of 152 studies reported elevated levels of cytokines IL (interleukin)-1β, IL-6, tumor necrosis factor (TNF), the chemokine CCL2 (MCP1), and C-reactive protein (CRP) in both peripheral blood and CSF of individuals with PD compared to healthy controls171. Conversely, CX3CL1 (fractalkine), CXCL12, soluble TNF receptor-1 (sTNFR1), and N-terminal-pro-B-type natriuretic peptide (NT-proBNP) were significantly increased only in peripheral blood, whereas nitric oxide was significantly elevated only in CSF171. Interferon (IFN)\(\alpha\)2, IFNγ, and IL-4 were significantly decreased in peripheral blood171. While these findings may reflect true differences between peripheral and central inflammation in PD, they could also be influenced by statistical power limitations (fewer studies on certain mediators) or technical variables such as assay sensitivity and timing of blood collection.
Although strenuous acute physical exercise can be pro-inflammatory172, chronic exercise consistently reduces inflammation across various populations, including individuals with type 2 diabetes, metabolic disorders, and aging-related conditions173,174,175,176. In these populations, exercise significantly lowers peripheral blood levels of TNF, IL-6, and CRP—biomarkers also elevated in PD. Small studies in individuals with PD have shown that moderate- to high-intensity interval training (8–12 weeks) significantly reduces TNF levels in peripheral blood, though no effect has been observed for IL-6, and CRP remains unexamined177,178. It is also unclear whether exercise-induced reductions in peripheral inflammation extend to the CNS. However, extensive preclinical evidence indicates that exercise reduces neuroinflammation, particularly IL-1β and TNF, in brain regions implicated in PD179, suggesting that similar mechanisms may apply to humans177,178,179.
Three primary reasons support the use of TNF, IL-6 and CRP as robust biomarkers for assessing the effects of exercise on inflammation in PD: 1) each of these three markers is significantly elevated in both peripheral blood and CSF in individuals with PD versus controls171; 2) evidence for their alteration in PD is stronger than for other inflammatory biomarkers, as these three mediators were analyzed in the largest number of studies (34–44 for peripheral blood, 8–14 for CSF)171; 3) exercise consistently lowers TNF, IL-6, and CRP levels across multiple populations with conditions relevant to PD173,174,175,176. While TNF, IL-6, and CRP appear to be the most reliable markers for studying exercise-induced changes in inflammation in PD, other factors—including IL-1β, CX3CL1, and clusterin—are emerging as relevant to both PD and exercise. Figure 3 presents a model depicting the role of these inflammatory markers in PD and the potential effects of exercise.
There are chronic increases in inflammatory markers in both central nervous system and periphery in PD. There is increased permeability of the BBB in PD, and many inflammatory markers including IL-6, TNF, IL-1β, and clusterin can cross the BBB, while others such as CRP and CX3CL1 modulate the permeability of the BBB in PD. Chronic aerobic exercise may be effective at reducing levels of inflammatory markers in both the central nervous system and periphery. PD Parkinson’s disease, BBB blood brain barrier, IL-6 interleukin-6, TNF tumor necrosis factor, IL-1β interleukin-1 beta, CRP c-reactive protein, CX3CL1, fractalkine. Created in BioRender. Mehta, N. (2025) https://BioRender.com/d17z951.
TNF
Evidence of elevated TNF protein levels or mRNA expression in the CSF, the striatum, and the substantia nigra of individuals with PD dates to 1994, marking the first reported cytokine alteration in PD180,181. Since then, extensive studies have examined TNF system dysregulation in both central and peripheral immune compartments of PD. Research indicates that innate and adaptive immune cells and microglia, produce higher TNF levels in individuals with PD compared to healthy controls182. Additionally, T lymphocytes in PD patients express higher TNF receptor levels, making them more susceptible to TNF’s biological effects182. Circulating TNF levels in both blood183,184,185 and CSF183,186,187 have consistently been reported as elevated in PD. Preclinical studies and epidemiological evidence suggests TNF plays a key role in PD pathogenesis188,189. In vitro and animal models of PD demonstrate TNF involvement in neuronal cell death182, while epidemiological studies reveal that early or mid-life exposure to anti-TNF therapy significantly reduces PD risk in individuals with IBD, a population already at elevated risk for PD190,191.
A recent meta-analysis by Khalafi et al. found that chronic aerobic exercise ( ≥ 7 weeks) significantly reduces soluble TNF levels in older adults176. Similarly, an earlier meta-analysis by Zheng et al. across five studies of chronic aerobic exercise ( ≥ 24 weeks) reported comparable reductions in blood TNF levels192. In individuals with PD, two small studies of moderate- and high-intensity interval training (8-12 weeks) have been shown to significantly lower soluble TNF levels in the blood177,178. Given that peripheral TNF alterations may reflect CNS changes in PD171, monitoring blood TNF levels in exercise trials could provide mechanistic insights into neuroinflammation while reducing reliance on invasive procedures.
IL-6
The cytokine IL-6 has been extensively studied in PD, with evidence indicating significantly elevated levels of IL-6 in both blood193,194 and CSF187,195 in individuals with PD compared to controls171. Additionally, serum IL-6 levels inversely correlate with measures of functional mobility, gait speed, and Mini-Mental Status Examination scores193,196. While studies have shown significant reductions in peripheral blood IL-6 levels in response to chronic exercise in individuals with various chronic inflammatory conditions173,174,176, trials assessing the effects of chronic aerobic exercise on IL-6 levels in people with PD remain lacking. Unlike TNF, which has a well-established detrimental role in PD, the impact of IL-6 in PD and in response to exercise is more complex197. IL-6 exhibits multiple signaling modalities and cellular targets, acting as both a pro- and anti-inflammatory mediator197,198. Preclinical studies indicate that IL-6 can promote both neuronal cell death and survival after injury, and it is also necessary for mediating the beneficial effects of exercise199. Consequently, evaluating the mechanistic response to exercise using IL-6 is more challenging than with other cytokines, as it requires assessing various components of the IL-6 system, including soluble IL-6 receptor levels, in addition to IL-6 itself.
CRP
As discussed in a recent perspective, the acute-phase protein CRP is the most studied biomarker of inflammation in PD and a key marker for evaluating the effects of exercise on inflammation200. A meta-analysis of 23 studies found that individuals with PD have significantly higher CRP levels both in the peripheral circulation and in the CSF compared to matched healthy controls201. Independent of disease duration or symptom severity, baseline CRP levels in PD participants are associated with an increased risk of death and poorer life prognosis202. Higher CRP levels also positively correlate with PD disease stage and motor symptoms203,204. In CSF, elevated CRP levels have also been linked to cognitive impairment, as well as increased severity of depression, anxiety, and fatigue in PD205. Multiple systematic reviews in middle-aged and older adults have shown that aerobic exercise, particularly at higher intensity and longer duration ( > 9 weeks), is associated with greater reductions in CRP levels176,192,206. However, no studies have yet examined the effects of aerobic exercise on CRP in individuals with PD. Although genetic data suggest that CRP is unlikely to play a direct role in PD pathogenesis207, CRP remains as a valuable biomarker for assessing overall inflammatory status in response to exercise. Unlike other mediators that are transiently expressed, CRP reflects the cumulative response of inflammatory cytokines and mediators. For further details, we refer the reader to our perspective200.
IL-1β
The pro-inflammatory cytokine IL-1β is elevated in both peripheral blood and CSF in individuals with PD compared to controls171. Various pathways, including aggregated α-synuclein, activate the NLRP3 inflammasome, a multi-molecular complex that processes pro-IL-1β into the mature, bioactive form, leading to neuroinflammation and neuronal death by pyroptosis208. Inhibition of IL-1β or NLRP3 is protective in preclinical models of PD208; however, no human studies have been conducted to date. While meta-analyses do not show a consistent reduction in IL-1β levels in response to exercise, a sub-analysis indicates that combining aerobic and resistance training significantly lowers IL-1β in individuals with overweight/obesity and cardiometabolic diseases209. The effects of aerobic exercise alone on IL-1β remain less clear, with only one study in obese women reporting a decrease in blood IL-1β following three months of aerobic exercise210. The uncertainty regarding exercise’s effectiveness in lowering IL-1β may stem from challenges in accurately quantifying this cytokine in peripheral blood, posing an additional hurdle for future research.
CX3CL1/Fractalkine
The chemokine CX3CL1 (fractalkine) has gained attention over the past decade as a biomarker of inflammation in PD and a potential link between PD and exercise211. A meta-analysis of 4 studies found a significant increase in CX3CL1 levels in peripheral blood of individuals with PD compared to controls, but no significant alterations in the CSF171. However, one study reported significantly reduced CSF levels in PD212. The effects of exercise on CX3CL1 remain inconclusive. CX3CL1 levels have been shown to decrease in young and middle-aged adults with mobility disabilities following 12 weeks of combined aerobic and resistance training213. Conversely, in a randomized controlled trial in adults with type 2 diabetes and coronary artery disease, a slight increase in circulating CX3CL1 levels was observed after 12 months of combined aerobic and resistance training214. In individuals with PD, 12 weeks of balance training significantly increased circulating CX3CL1 levels215, but the effects of aerobic exercise training remain unknown. The limited number of studies examining the impact of exercise on CX3CL1 levels, along with conflicting findings in both PD and exercise research, highlights the need for further investigation to determine the utility of CX3CL1 as a biomarker of inflammation in exercise interventions in PD.
Clusterin
Clusterin is an emerging biomarker produced by hepatocytes in response to exercise and may play a role in modulating inflammation142,216. As an extracellular chaperone, clusterin exerts anti-inflammatory effects primarily by suppressing complement activation and preventing or slowing the aggregation of misfolded proteins, including α-synuclein, tau, and amyloid-beta217,218. De Miguel et al. demonstrated that plasma collected from voluntarily running mice and infused into sedentary mice reduces neuroinflammatory gene expression and brain inflammation216. However, immunodepletion of clusterin abolished these anti-inflammatory benefits216. Additionally, in a small cohort of veterans with MCI, six months of combined aerobic and resistance training led to increased plasma clusterin levels216. An exercise-induced increase in clusterin may be particularly relevant to PD, as studies have shown that exosomes isolated from the plasma of individuals with PD at Hoehn and Yahr stage II exhibit significantly lower clusterin expression compared to healthy individuals219. While further research is needed to confirm the effects of exercise on clusterin in PD, current evidence suggests that aerobic exercise may help increase clusterin levels, potentially offering neuroprotective benefits.
Exercise-Induced Changes in Neuroendocrine Markers
Neuroendocrine abnormalities have been reported at all stages of PD and are associated with multiple motor and non-motor symptoms220,221. Mounting evidence suggests these abnormalities are an intrinsic feature of PD rather than a consequence of other physiological processes or medication side effects. The neuroendocrine system integrates endocrine outputs from the nervous system and peripheral hormones that influence brain function. Neuroendocrine changes in PD affect various physiological functions, including stress response (cortisol), circadian rhythm (melatonin), insulin resistance (insulin), bone metabolism (vitamin D), and aging (klotho). Disruptions in these pathways or altered hormone levels have been linked to increased risk of PD. For example, epidemiological studies associate increased stress222,223, circadian rhythm dysfunction224,225, type 2 diabetes226,227, vitamin D deficiency228,229,230, and aging231 with a higher risk of developing PD.
Most neuroendocrine abnormalities in PD have primarily been identified in blood or saliva, with fewer studies assessing CSF. Cortisol levels are consistently elevated in blood232,233,234 and saliva235,236 of individuals with PD. Conversely, melatonin, insulin, and vitamin D levels are typically reduced in PD, though CSF studies for these markers remain limited237,238,239,240. Klotho, a longevity-related hormone, is significantly lower in the CSF of individuals with PD compared to healthy controls, but findings in blood are inconsistent241. While Kakar et al. reported no change in plasma klotho levels in moderate to advanced PD242, Sanscessario et al. found lower serum klotho levels in early-stage PD243. These findings suggest that hormone levels may vary with disease stage and may reflect distinct peripheral and CNS pools.
Exercise has the potential to modify the neuroendocrine abnormalities associated with PD. Chronic exercise training reduces hormonal stress responses, lowering cortisol levels108,244. It also enhances melatonin secretion245,246, improves insulin sensitivity both peripherally and in the CNS247,248, and increases circulating vitamin D249 and klotho levels250,251,252. Among these neuroendocrine markers, cortisol, insulin, and klotho may serve as robust biomarkers for assessing exercise benefits in PD because: 1) they exhibit consistent abnormalities in PD; 2) they play key roles in PD-related pathophysiological pathways; and 3) they have been shown to respond significantly to exercise (Fig. 4).
In PD, there is persistent elevation of stress hormone cortisol, increased insulin resistance, and reduction in klotho levels. These abnormalities have been linked to increased neuroinflammation, oxidative stress, neurodegeneration, and aging. Chronic aerobic exercise can help lower blood cortisol levels, reduce insulin resistance, and increase blood klotho levels, which may counter these pathological pathways. While cortisol and insulin can cross the BBB, klotho cannot cross the BBB and has two distinct pools (CNS and periphery). PD Parkinson’s disease, BBB blood brain barrier, CNS central nervous system. Created in BioRender. Mehta, N. (2025) https://BioRender.com/j77o278.
Cortisol
Cortisol has widespread effects on the brain, affecting mood, behavior, cognition, and stress response regulation253. Stress modulates motor system function, as most parts of the motor regions express glucocorticoid receptors, the primary targets for cortisol254. In PD mouse models, chronic stress exacerbates motor deficits, accelerates nigrostriatal neurodegeneration, and impairs compensatory motor recovery255. In individuals with PD, cortisol levels have been shown to correlate with severity of depression, prevalence of anxiety and anhedonia, longer sleep latency, and increased risk-taking behavior236,254,256,257. However, the relationship between cortisol and PD motor symptoms remains inconsistent. While Haglin et al. found higher cortisol levels correlated with greater motor symptom severity (as measured by MDS-UPDRS motor scores)258, Muller and Muhlack reported significantly lower cortisol levels in individuals at advanced PD stages compared to those in earlier stages259. Interestingly, acute levodopa administration has been shown to decrease cortisol levels, correlating with motor symptom improvement, suggesting that interventions lowering cortisol may help alleviate PD symptoms259.
Chronic exercise training induces neuroendocrine adaptations that ultimately reduce cortisol levels260. In women with MCI, 6 months of high-intensity aerobic exercise reduced plasma cortisol levels and improved executive cognitive function108. A meta-analysis by Beserra et al. found that regular exercise reduces daytime cortisol levels in individuals with major depressive disorder, with aerobic and more frequent exercise yielding greater effects244. While research on exercise and cortisol regulation in PD is limited, Smyth et al. demonstrated that high-intensity treadmill training reduced cortisol secretion during the post-awakening period in individuals with PD261. These findings suggest that cortisol may serve as a promising biomarker for assessing exercise response and its impact on PD symptoms.
Insulin
Insulin plays a central role in peripheral glucose metabolism, but within the central nervous system, it also regulates dopaminergic transmission, maintenance of synapses, synaptic plasticity, and neuronal survival and growth262. In PD, both insulin deficiency and insulin resistance contribute to impaired brain insulin signaling which may drive neuroinflammation, mitochondrial dysfunction, and oxidative stress237,263,264,265,266. In rodent models, insulin resistance has been linked to reduced surface expression of dopamine transporters in the striatum, increased ROS, and α-synuclein aberrant expression267,268,269,270. Induction of insulin signaling with IGF-1 and reversal of insulin resistance has been shown to suppress α-synuclein aggregation and toxicity in cultured cells271. Insulin resistance in individuals with PD associates with a more severe phenotype, accelerated disease progression, and increased risk of cognitive decline, whereas individuals treated with antidiabetic drugs have a lower risk of developing PD272. Several antidiabetic drugs, i.e. exenatide and lixisenatide, are being explored as potential disease-modifying agents for PD273,274.
Exercise is well known to improve insulin sensitivity peripherally248,275, and has more recently been shown to positively modulate brain insulin signaling pathways276,277. In rodent models of memory impairment, exercise improves insulin signaling alongside cognitive function278. In middle-aged sedentary individuals, exercise for 8 weeks increases brain insulin sensitivity following intranasal insulin administration279. Exercise also upregulates IGF-1 gene expression and protein levels in several brain regions, especially those involved in learning and cognition280. Additionally, it increases circulating IGF-1 in the periphery, which can cross the BBB and reach the brain281. Given the role of insulin resistance in PD pathogenesis and the ability of exercise to mitigate it, insulin may serve as a valuable biomarker for assessing exercise responsiveness in individuals with PD.
Klotho
α-Klotho (hereafter referred to as klotho) is a pleiotropic protein recognized as a master regulator of aging. In mice, klotho deficiency leads to a significantly shortened lifespan and premature aging, while its overexpression extends lifespan by approximately 30%282,283. Klotho functions both as a membrane-bound coreceptor for fibroblast growth factor 23 and as a circulatory endocrine mediator exhibiting anti-inflammatory, antioxidant, and neuroprotective properties284,285. Klotho inhibits multiple aging-related pathways, including transforming growth factor β, IGF-1, Wnt and NF-κB282,286,287,288. In the context of PD, klotho overexpression or exogenous administration protects dopaminergic neurons against oxidative injury and alleviates astrogliosis, apoptosis, and oxidative stress289,290. Additionally, it improves cognition, motor function, and synaptic plasticity in PD mouse models289. In humans, higher klotho levels have been associated with increased lifespan and enhanced cognition in aging populations291,292,293. Furthermore, elevated klotho levels correlate with reduced amyloid-beta burden and improved cognition in individuals at risk for Alzheimer’s disease (AD)294.
Recent studies have linked klotho to PD progression. CSF klotho levels have been shown to be lower in individuals with moderate stage PD compared to controls, correlating with greater motor impairment, higher Hoehn and Yahr disability stages, and poorer cognitive performance241,295,296. Plasma klotho levels appear unchanged in moderately advanced PD, although women with PD exhibit higher levels than men242. Interestingly, one study found the opposite pattern in early stages of PD, with higher CSF levels of klotho and lower serum klotho levels243. These discrepancies may stem from differences in assay methodologies or disease stage. Like IGF-1 dynamics, an initial compensatory increase in CSF klotho may occur in early PD but decline as the disease progresses103,104,105,106.
Beyond neuroprotection, klotho is associated with better lower extremity strength and physical function in older adults297,298. Higher klotho levels have also been observed in physically trained individuals compared to their sedentary counterparts299. Chronic aerobic training increases plasma klotho levels in healthy middle-aged adults and older women250,251,300. A recent meta-analysis confirmed that at least 12 weeks of aerobic exercise significantly elevates circulating klotho levels in both healthy individuals and those with chronic diseases252. However, no studies have yet examined how exercise influences klotho levels in individuals with PD. Mechanistically, exercise may upregulate klotho via myokines such as irisin. Jin et al. demonstrated that irisin enhances cognition and reduces mortality in mice following cerebral ischemia through klotho upregulation301. Notably, these benefits were abolished in klotho-knockout mice, underscoring klotho’s critical role in exercise-mediated neuroprotection. Future research should explore how klotho responds to exercise in people with PD and whether its modulation correlates with symptom progression and disease severity.
Exercise-Induced Changes in Markers of PD Pathology
α-Synuclein
Exercise is likely to affect PD pathogenesis and risk. Among candidate biomarkers of PD pathology, α-synuclein is a key protein, as it constitutes the major component of Lewy bodies, the pathological hallmark of PD302. α -Synuclein is highly conserved within mammals and widely expressed in the brain and throughout the body, including the myocardium, skin fibroblasts, saliva, muscle, bone marrow, liver, and spleen303. It can cross the BBB bidirectionally, moving between the blood and the brain. In the brain, α-synuclein plays a crucial role at presynaptic sites, where it regulates neurotransmitter release and reuptake, synaptic vesicle trafficking, and exerts chaperone-like activity304. In PD, α-synuclein misfolds into oligomers and amyloid fibrils, adopting a β-sheet conformation.
α-Synuclein centrality to PD is further underscored by mutations in the SNCA gene, which cause rare familial forms of PD305,306, and genetic variants of α-synuclein that increase the risk of sporadic PD307. Although total α-synuclein levels in the CSF are slightly lower in PD participants compared to controls, values largely overlap at the population level308. A major recent breakthrough in PD diagnostics is the α-synuclein seed amplification assay (SAA), also known as real-time quaking-induced conversion (RT-QuIC) or protein misfolding cyclic amplification assay (PMCA). This assay detects misfolded α-synuclein with high diagnostic accuracy (88-97% sensitivity and 90-99% specificity) when performed on CSF309,310,311,312,313,314,315,316,317,318,319,320,321.
Notably, studies of prodromal PD also show a high positivity rate for α-synuclein SAA315,319,320,321, indicating that misfolded α-synuclein can be detected decades before clinical diagnosis, even before dopaminergic neurodegeneration becomes apparent on brain imaging320. To facilitate wider clinical application, α-synuclein SAA is being explored in more accessible biofluids and tissues, such as skin biopsies322,323,324, olfactory epithelium325, saliva326,327, blood extracellular vesicles328 and in serum329. While initial results, especially from skin biopsies, are promising, the reliability and accuracy of SAA using these peripheral sources require further validation through independent studies.
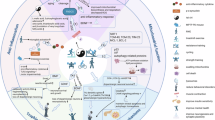
Although clinical evidence linking exercise and α-synuclein is currently lacking, preclinical studies provide insight into this relationship. In rodent models of PD, mild- to moderate-intensity treadmill exercise (5 days a week for 6-8 weeks) has been associated with reduced α-synuclein levels in striatum and substantia nigra, alongside improvements in motor function330,331,332,333,334. As clinical research advances, future studies will help elucidate how exercise influences α-synuclein pathology in PD and how to measure its potential changes with quantitative assays. Figure 5 proposes a model illustrating how exercise-induced biomarkers may modulate pathological α-synuclein.
Exercise may induce increases in irisin, clusterin, and SIRT3, markers that have been shown to reduce conversion of misfolded α- synuclein to pathological α-synuclein. Pathological α-synuclein aggregate formation in PD can lead to oxidative stress, mitochondrial dysfunction, pro-inflammatory cytokine release, and neuronal degeneration. Insulin resistance also associates with increased α-synuclein aggregate formation, but exercise may decrease insulin resistance and thus reduce aggregated protein. Axonal injury in PD releases NfL with preliminary evidence suggesting exercise may lower circulating NfL levels. α-syn, α-synuclein; SIRT3 sirtuin-3, NfL neurofilament light chain. Created in BioRender. Mehta, N. (2025) https://BioRender.com/h90y253.
Neurofilament light chain
Neurofilament light chain (NfL) is a promising biomarker of disease severity and progression in PD. However, it lacks specificity, as elevated NfL levels are observed in multiple neurodegenerative disorders335,336. NfL is a structural component of the neuronal cytoskeleton, released into biofluids following axonal damage in neurodegenerative disorders, neuroinflammatory conditions, traumatic nervous system injury, stroke, and during normal aging. Serum or plasma NfL correlate with CSF levels337,338 making it a more accessible biomarker than CSF sampling. Despite its nonspecific nature, NfL shows potential for monitoring disease progression, prognosis, and treatment response338. Whether NfL can serve as an exercise response biomarker remains unclear due to limited research. In MS, an 8-week aerobic training program lowered serum NfL levels339, whereas a 16-week aerobic training program in mild AD patients showed no significant changes340. In a toxin-induced hemiparkinsonian rat model, moderate-intensity aerobic exercise reduced serum NfL levels341. To date, no clinical studies have examined NfL changes in response to exercise in individuals with PD. Further studies are needed to determine whether exercise influences NfL levels in PD and if it could serve as a reliable marker for neuroprotection or disease modification.
Exercise-Induced Changes in Other Diseases of the Nervous System
The findings summarized in this review show striking similarities to those observed in other diseases of the central nervous system. In AD, large-scale transcriptomic analyses have identified exercise and physical activity as the top theoretical treatments, reversing the expression patterns of hundreds of AD-related genes across multiple functional categories342. While similar gene expression analyses have not been conducted in PD, comparable outcomes are expected. A review by Paillard, Blain and Bernard examined the effects of exercise in AD, similar to the approach taken in this paper343. They concluded that aerobic exercise promotes angiogenesis and the release of neurotrophic factors (e.g., IGF-1, BDNF, VEGF), which enhance cerebral blood flow and support neuroplasticity. Additionally, IGF-1 is believed to facilitate amyloid-beta clearance and reduce tau hyperphosphorylation, both of which are central to AD pathology. Exercise has also been shown to provide significant benefits in MS344. However, unlike PD and AD, no fluid biomarkers have yet emerged as clear indicators of exercise-induced therapeutic effects in MS344.
Conclusion
Chronic aerobic exercise induces multidimensional changes by modulating various pathways involved in the development and progression of PD. In this review, we have identified BDNF, GDNF, IGF-1, VEGF, TNF, IL-6, CRP, cortisol, insulin, and klotho as candidate biomarkers with the strongest evidence for their responsiveness to aerobic exercise and potential roles in neuroprotection and symptom alleviation in PD. Additionally, emerging evidence suggests that irisin, GPLD1, SIRT3, IL-1β, CX3CL1, and clusterin may also play significant roles in PD and exercise adaptation. Table 1 summarizes all biomarkers. Currently, there is limited data on how exercise affects markers of PD pathology, including α-synuclein and NfL, particularly in the prodromal and early disease stages. Future studies should explore whether exercise influences these biomarkers and their relationship with disease progression.
Many of the biomarkers identified likely mediate bidirectional crosstalk between the periphery and the brain, driving exercise-induced neuroprotection. Tracking these biomarkers in exercise intervention studies will enhance our mechanistic understanding of how chronic aerobic exercise benefits individuals with PD. Furthermore, identifying a biofluid profile that reliably responds to exercise would facilitate comparisons between different exercise intensities and modalities to determine optimal dosage effects. One such study, SPARX3, aims to evaluate the effects of two different aerobic exercise intensities on these biomarkers in people with early-stage PD44. It will also be essential to analyze associations between specific biomarkers and motor/non-motor PD symptoms as well as disease progression. Once a validated panel of exercise response biomarkers is established, these markers could potentially be used in clinical settings to track an individual’s response to exercise and tailor regimens to elicit optimal molecular responses for symptom management or disease modification. However, factors such as sensitivity/specificity, cost, assay variability, and feasibility of routine biomarker testing must be considered for clinical application.
Our review has several limitations: 1) Biomarker selection is based on a literature review of markers implicated in PD and linked to exercise but may not include all responsive markers; 2) it focuses on aerobic exercise without comparing effects across different exercise modalities (e.g., resistance training); and 3) the studies reviewed vary in quality and sample sizes (see Supplementary Information), limiting generalizability. While this review primarily addresses chronic aerobic exercise, future research should also investigate acute exercise effects on these biomarkers. For instance, lactate, cortisol, and inflammatory markers typically increase immediately after acute exercise, contrasting with their long-term reductions observed with chronic exercise. Understanding how acute vs. chronic biomarker changes impact PD symptoms and disease mechanisms will be crucial for optimizing exercise-based interventions.
References
Hirtz, D. et al. How common are the “common” neurologic disorders?. Neurology 68, 326–337 (2007).
Dorsey, E. R. & Bloem, B. R. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 75, 9–10 (2018).
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 896–912 (2015).
Vijiaratnam, N., Simuni, T., Bandmann, O., Morris, H. R. & Foltynie, T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 20, 559–572 (2021).
Lang, A. E. & Espay, A. J. Disease Modification in Parkinson’s Disease: Current Approaches, Challenges, and Future Considerations. Mov. Disord. 33, 660–677 (2018).
Tanner, C. M. & Ostrem, J. L. Parkinson’s Disease. N. Engl. J. Med 391, 442–452 (2024).
Jankovic, J. & Tan, E. K. Parkinson’s disease: etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 91, 795–808 (2020).
Blesa, J., Foffani, G., Dehay, B., Bezard, E. & Obeso, J. A. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first. Nat. Rev. Neurosci. 23, 115–128 (2022).
Schapira, A. H. & Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 26, 1049–1055 (2011).
Pajares, M., A, I. R., Manda, G., Bosca, L. & Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 9, https://doi.org/10.3390/cells9071687 (2020).
Rocha, E. M., De Miranda, B. & Sanders, L. H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 109, 249–257 (2018).
de Almeida, F. O., Santana, V., Corcos, D. M., Ugrinowitsch, C. & Silva-Batista, C. Effects of Endurance Training on Motor Signs of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Sports Med 52, 1789–1815 (2022).
Gollan, R. et al. Effects of Resistance Training on Motor- and Non-Motor Symptoms in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Parkinson’s Dis.https://doi.org/10.3233/jpd-223252 (2022).
Jin, X. et al. The Impact of Mind-body Exercises on Motor Function, Depressive Symptoms, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-analysis. Int. J. Environ. Res. Public Health 17, https://doi.org/10.3390/ijerph17010031 (2019).
Liu, H. H. et al. Effects of Tai Chi Exercise on Reducing Falls and Improving Balance Performance in Parkinson’s Disease: A Meta-Analysis. Parkinsons Dis. 2019, 9626934 (2019).
Aras, B., Seyyar, G. K., Fidan, O. & Colak, E. The effect of Tai Chi on functional mobility, balance and falls in Parkinson’s disease: A systematic review and meta-analysis of systematic reviews. Explor. (NY) 18, 402–410 (2022).
Wu, C. et al. Effects of Aerobic Exercise and Mind-Body Exercise in Parkinson’s Disease: A Mixed-Treatment Comparison Analysis. Front. aging Neurosci. 13, 739115 (2021).
Ernst, M. et al. Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database Syst. Rev. 1, CD013856 (2023).
Corcos, D. M., Lamotte, G., Luthra, N. S. & McKee, K. E. Advice to People with Parkinson’s in My Clinic: Exercise. J. Parkinsons Dis. 14, 609–617 (2024).
Schenkman, M. et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 75, 219–226 (2018).
van der Kolk, N. M. et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 18, 998–1008 (2019).
Mak, M. K. Y. & Wong-Yu, I. S. K. Six-Month Community-Based Brisk Walking and Balance Exercise Alleviates Motor Symptoms and Promotes Functions in People with Parkinson’s Disease: A Randomized Controlled. Trial J. Parkinsons Dis. 11, 1431–1441 (2021).
Hou, L., Chen, W., Liu, X., Qiao, D. & Zhou, F. M. Exercise-Induced Neuroprotection of the Nigrostriatal Dopamine System in Parkinson’s Disease. Front Aging Neurosci. 9, 358 (2017).
Paul, K. C. et al. The association between lifestyle factors and Parkinson’s disease progression and mortality. Mov. Disord. 34, 58–66 (2019).
Combs-Miller, S. A. & Moore, E. S. Predictors of outcomes in exercisers with Parkinson disease: A two-year longitudinal cohort study. NeuroRehabilitation 44, 425–432 (2019).
Oguh, O., Eisenstein, A., Kwasny, M. & Simuni, T. Back to the basics: regular exercise matters in parkinson’s disease: results from the National Parkinson Foundation QII registry study. Parkinsonism Relat. Disord. 20, 1221–1225 (2014).
Sasco, A. J., Paffenbarger, R. S. Jr., Gendre, I. & Wing, A. L. The role of physical exercise in the occurrence of Parkinson’s disease. Arch. Neurol. 49, 360–365 (1992).
Yang, F. et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain 138, 269–275 (2015).
de Laat, B. et al. Intense exercise increases dopamine transporter and neuromelanin concentrations in the substantia nigra in Parkinson’s disease. NPJ Parkinsons Dis. 10, 34 (2024).
Altmann, L. J. et al. Aerobic Exercise Improves Mood, Cognition, and Language Function in Parkinson’s Disease: Results of a Controlled Study. J. Int Neuropsychol. Soc. 22, 878–889 (2016).
Silveira, C. R. A., Roy, E. A., Intzandt, B. N. & Almeida, Q. J. Aerobic exercise is more effective than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain Cogn. 122, 1–8 (2018).
van der Kolk, N. M. et al. A remotely supervised home-based aerobic exercise programme is feasible for patients with Parkinson’s disease: results of a small randomised feasibility trial. J. Neurol., Neurosurg., psychiatry 89, 1003–1005 (2018).
Schootemeijer, S., van der Kolk, N. M., Bloem, B. R. & de Vries, N. M. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. NeuroTherapeutics: J. Am. Soc. Exp. NeuroTherapeutics 17, 1418–1433 (2020).
Crowley, E. K., Nolan, Y. M. & Sullivan, A. M. Exercise as a therapeutic intervention for motor and non-motor symptoms in Parkinson’s disease: Evidence from rodent models. Prog. Neurobiol. 172, 2–22 (2019).
Amara, A. W. & Memon, A. A. Effects of Exercise on Non-motor Symptoms in Parkinson’s Disease. Clin. Ther. 40, 8–15 (2018).
Pfeiffer, R. F. Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 22, S119–S122 (2016).
in BEST (Biomarkers, EndpointS, and other Tools) Resource (2016).
Zigmond, M. J. et al. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. Disord. 15, S42–S45 (2009).
Tajiri, N. et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res 1310, 200–207 (2010).
Tuon, T. et al. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 227, 305–312 (2012).
Cotman, C. W., Berchtold, N. C. & Christie, L. A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472 (2007).
Sujkowski, A., Hong, L., Wessells, R. J. & Todi, S. V. The protective role of exercise against age-related neurodegeneration. Ageing Res Rev. 74, 101543 (2022).
Monteiro-Junior, R. S. et al. We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses 85, 537–541 (2015).
Patterson, C. G. et al. Study in Parkinson’s disease of exercise phase 3 (SPARX3): study protocol for a randomized controlled trial. Trials 23, 855 (2022).
Dickson, D. W. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb. Perspect. Med. 2, https://doi.org/10.1101/cshperspect.a009258 (2012).
Tome, D., Fonseca, C. P., Campos, F. L. & Baltazar, G. Role of Neurotrophic Factors in Parkinson’s Disease. Curr. Pharm. Des. 23, 809–838 (2017).
Ferreira, R. N. et al. Neurotrophic Factors in Parkinson’s Disease: What Have we Learned from Pre-Clinical and Clinical Studies?. Curr. Med Chem. 25, 3682–3702 (2018).
Paul, G. & Sullivan, A. M. Trophic factors for Parkinson’s disease: Where are we and where do we go from here. Eur. J. Neurosci. 49, 440–452 (2019).
Sullivan, A. M. & Toulouse, A. Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine Growth Factor Rev. 22, 157–165 (2011).
da Silva, P. G., Domingues, D. D., de Carvalho, L. A., Allodi, S. & Correa, C. L. Neurotrophic factors in Parkinson’s disease are regulated by exercise: Evidence-based practice. J. Neurol. Sci. 363, 5–15 (2016).
Parain, K. et al. Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. Neuroreport 10, 557–561 (1999).
Nagatsu, T. & Sawada, M. Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J. Neural Transm. Suppl. 113–120, (2007).
Nagatsu, T. Parkinson’s disease: changes in apoptosis-related factors suggesting possible gene therapy. J. Neural Transm. (Vienna) 109, 731–745 (2002).
Virachit, S. et al. Levels of glial cell line-derived neurotrophic factor are decreased, but fibroblast growth factor 2 and cerebral dopamine neurotrophic factor are increased in the hippocampus in Parkinson’s disease. Brain Pathol. 29, 813–825 (2019).
Chauhan, N. B., Siegel, G. J. & Lee, J. M. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J. Chem. Neuroanat. 21, 277–288 (2001).
Janelidze, S. et al. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology 85, 1834–1842 (2015).
Jiang, L. et al. Serum level of brain-derived neurotrophic factor in Parkinson’s disease: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 88, 168–174 (2019).
Lorigados Pedre, L. et al. Nerve growth factor levels in Parkinson disease and experimental parkinsonian rats. Brain Res 952, 122–127 (2002).
Tong, S. Y. et al. Serum glial cell line-derived neurotrophic factor (GDNF) a potential biomarker of executive function in Parkinson’s disease. Front Neurosci. 17, 1136499 (2023).
Rahmani, F. et al. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain Res 1704, 127–136 (2019).
Mashayekhi, F., Mirzajani, E., Naji, M. & Azari, M. Expression of insulin-like growth factor-1 and insulin-like growth factor binding proteins in the serum and cerebrospinal fluid of patients with Parkinson’s disease. J. Clin. Neurosci. 17, 623–627 (2010).
Galli, E. et al. Increased Serum Levels of Mesencephalic Astrocyte-Derived Neurotrophic Factor in Subjects With Parkinson’s Disease. Front Neurosci. 13, 929 (2019).
Bonanni, R., Cariati, I., Tarantino, U., D’Arcangelo, G. & Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 7, https://doi.org/10.3390/jfmk7020038 (2022).
da Silva, W. A. B. et al. Physical exercise increases the production of tyrosine hydroxylase and CDNF in the spinal cord of a Parkinson’s disease mouse model. Neurosci. Lett. 760, 136089 (2021).
Kowianski, P. et al. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 38, 579–593 (2018).
Porritt, M. J., Batchelor, P. E. & Howells, D. W. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp. Neurol. 192, 226–234 (2005).
Lucidi-Phillipi, C. A. et al. Brain-derived neurotrophic factor-transduced fibroblasts: production of BDNF and effects of grafting to the adult rat brain. J. Comp. Neurol. 354, 361–376 (1995).
Levivier, M., Przedborski, S., Bencsics, C. & Kang, U. J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 15, 7810–7820 (1995).
Howells, D. W. et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp. Neurol. 166, 127–135 (2000).
Wang, Y., Liu, H., Zhang, B. S., Soares, J. C. & Zhang, X. Y. Low BDNF is associated with cognitive impairments in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 29, 66–71 (2016).
Huang, Y., Huang, C., Zhang, Q., Wu, W. & Sun, J. Serum BDNF discriminates Parkinson’s disease patients with depression from without depression and reflect motor severity and gender differences. J. Neurol. 268, 1411–1418 (2021).
Scalzo, P., Kummer, A., Bretas, T. L., Cardoso, F. & Teixeira, A. L. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 257, 540–545 (2010).
Renteria, I. et al. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front Physiol. 13, 934714 (2022).
Bastioli, G. et al. Voluntary Exercise Boosts Striatal Dopamine Release: Evidence for the Necessary and Sufficient Role of BDNF. J. Neurosci. 42, 4725–4736 (2022).
Liu, P. Z. & Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci. 12, 52 (2018).
Vaynman, S., Ying, Z. & Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590 (2004).
Lau, Y. S., Patki, G., Das-Panja, K., Le, W. D. & Ahmad, S. O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur. J. Neurosci. 33, 1264–1274 (2011).
Szuhany, K. L., Bugatti, M. & Otto, M. W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res 60, 56–64 (2015).
Gholami, F., Mesrabadi, J., Iranpour, M. & Donyaei, A. Exercise training alters resting brain-derived neurotrophic factor concentration in older adults: A systematic review with meta-analysis of randomized-controlled trials. Exp. Gerontol. 199, 112658 (2025).
Rotondo, R. et al. Physical activity and neurotrophic factors as potential drivers of neuroplasticity in Parkinson’s Disease: A systematic review and meta-analysis. Ageing Res Rev. 92, 102089 (2023).
Paterno, A., Polsinelli, G. & Federico, B. Changes of brain-derived neurotrophic factor (BDNF) levels after different exercise protocols: a systematic review of clinical studies in Parkinson’s disease. Front Physiol. 15, 1352305 (2024).
Kaagman, D. G. M. et al. Effects and Mechanisms of Exercise on Brain-Derived Neurotrophic Factor (BDNF) Levels and Clinical Outcomes in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Brain Sci. 14, https://doi.org/10.3390/brainsci14030194 (2024).
Liu, Y., Tong, S., Ding, L., Liu, N. & Gao, D. Serum levels of glial cell line-derived neurotrophic factor and multiple neurotransmitters: In relation to cognitive performance in Parkinson’s disease with mild cognitive impairment. Int J. Geriatr. Psychiatry 35, 153–162 (2020).
Kirik, D., Rosenblad, C. & Bjorklund, A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur. J. Neurosci. 12, 3871–3882 (2000).
Winkler, C., Sauer, H., Lee, C. S. & Bjorklund, A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 16, 7206–7215 (1996).
Zhang, Z. et al. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J. Pharm. Exp. Ther. 282, 1396–1401 (1997).
Gill, S. S. et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med 9, 589–595 (2003).
Slevin, J. T. et al. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 102, 216–222 (2005).
Lang, A. E. et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466 (2006).
Whone, A. et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142, 512–525 (2019).
Whone, A. L. et al. Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J. Parkinsons Dis. 9, 301–313 (2019).
Speck, A. E. et al. Treadmill Exercise Attenuates L-DOPA-Induced Dyskinesia and Increases Striatal Levels of Glial Cell-Derived Neurotrophic Factor (GDNF) in Hemiparkinsonian Mice. Mol. Neurobiol. 56, 2944–2951 (2019).
McCullough, M. J., Gyorkos, A. M. & Spitsbergen, J. M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 240, 258–268 (2013).
Gyorkos, A. M., McCullough, M. J. & Spitsbergen, J. M. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience 257, 111–118 (2014).
Rabelo, P. C. R. et al. Intrinsic exercise capacity in rats influences dopamine neuroplasticity induced by physical training. J. Appl Physiol. (1985) 123, 1721–1729 (2017).
Aguiar, A. S. Jr. et al. Effects of exercise on mitochondrial function, neuroplasticity and anxio-depressive behavior of mice. Neuroscience 271, 56–63 (2014).
Kong, L., Miu, L., Yao, W. & Shi, Z. Effect of Regular Aerobic Exercise on Cognitive Function, Depression Level and Regulative Role of Neurotrophic Factor: A Prospective Cohort Study in the Young and the Middle-Aged Sample. Risk Manag Health. Policy 17, 935–943 (2024).
Wrigley, S., Arafa, D. & Tropea, D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front Cell Neurosci. 11, 14 (2017).
Lopez-Lopez, C., LeRoith, D. & Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl Acad. Sci. USA 101, 9833–9838 (2004).
Carro, E., Trejo, J. L., Gomez-Isla, T., LeRoith, D. & Torres-Aleman, I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat. Med 8, 1390–1397 (2002).
Ding, Q., Vaynman, S., Akhavan, M., Ying, Z. & Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140, 823–833 (2006).
Trejo, J. L., Carro, E. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 (2001).
Castilla-Cortazar, I., Aguirre, G. A., Femat-Roldan, G., Martin-Estal, I. & Espinosa, L. Is insulin-like growth factor-1 involved in Parkinson’s disease development?. J. Transl. Med 18, 70 (2020).
Shi, X. et al. Correlation between serum IGF-1 and EGF levels and neuropsychiatric and cognitive in Parkinson’s disease patients. Neurol. Sci. 44, 881–887 (2023).
Picillo, M. et al. Serum IGF-1 is associated with cognitive functions in early, drug-naive Parkinson’s disease. PLoS One 12, e0186508 (2017).
Godau, J., Herfurth, M., Kattner, B., Gasser, T. & Berg, D. Increased serum insulin-like growth factor 1 in early idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 81, 536–538 (2010).
Pellecchia, M. T. et al. Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naive Parkinson’s disease. Eur. J. Neurol. 21, 802–807 (2014).
Baker, L. D. et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 67, 71–79 (2010).
Nasir, Y., Hoseinipouya, M. R., Eshaghi, H. & Rahimi, M. H. The impact of exercise on growth factors in postmenopausal women: a systematic review and meta-analysis. BMC Women’s Health 24, 396 (2024).
Baker, L. D. et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J. Alzheimers Dis. 22, 569–579 (2010).
Rahe, J. et al. Cognitive training with and without additional physical activity in healthy older adults: cognitive effects, neurobiological mechanisms, and prediction of training success. Front Aging Neurosci. 7, 187 (2015).
Maass, A. et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage 131, 142–154 (2016).
Voss, M. W. et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav. Immun. 28, 90–99 (2013).
Stuckenschneider, T. et al. Disease-inclusive exercise classes improve physical fitness and reduce depressive symptoms in individuals with and without Parkinson’s disease-A feasibility study. Brain Behav. 11, e2352 (2021).
Rosenstein, J. M., Krum, J. M. & Ruhrberg, C. VEGF in the nervous system. Organogenesis 6, 107–114 (2010).
Fabel, K. et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812 (2003).
Yasuhara, T. et al. Neurorescue effects of VEGF on a rat model of Parkinson’s disease. Brain Res 1053, 10–18 (2005).
Yasuhara, T. et al. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur. J. Neurosci. 19, 1494–1504 (2004).
Infante, J., Mateo, I., Rodriguez-Rodriguez, E., Berciano, J. & Combarros, O. VEGF serum levels are not associated with Parkinson’s disease. Eur. J. Neurol. 14, e6 (2007).
Faucheux, B. A., Bonnet, A. M., Agid, Y. & Hirsch, E. C. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet 353, 981–982 (1999).
Barcia, C. et al. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J. Neural Transm. (Vienna) 112, 1237–1248 (2005).
Fowler, A. J. et al. CSF MicroRNAs Reveal Impairment of Angiogenesis and Autophagy in Parkinson Disease. Neurol. Genet 7, e633 (2021).
Gao, Y. et al. Treadmill exercise promotes angiogenesis in the ischemic penumbra of rat brains through caveolin-1/VEGF signaling pathways. Brain Res 1585, 83–90 (2014).
Morland, C. et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8, 15557 (2017).
Villar-Cheda, B. et al. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson’s disease. J. Cereb. Blood Flow. Metab. 29, 230–234 (2009).
Song, B. X. et al. The effect of exercise on blood concentrations of angiogenesis markers in older adults: A systematic review and meta-analysis. Neurobiol. Aging 135, 15–25 (2024).
Bostrom, P. et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Zhang, Y., Wang, L., Kang, H., Lin, C. Y. & Fan, Y. Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms24076551 (2023).
Qi, J. Y. et al. Irisin: A promising treatment for neurodegenerative diseases. Neuroscience 498, 289–299 (2022).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Wrann, C. D. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 1, 55–61 (2015).
Liu, Y., Zhu, C., Guo, J., Chen, Y. & Meng, C. The Neuroprotective Effect of Irisin in Ischemic Stroke. Front Aging Neurosci. 12, 588958 (2020).
Wang, Y. et al. Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin alphaVbeta5/AMPK signaling pathway after intracerebral hemorrhage in mice. J. Neuroinflammation 19, 82 (2022).
Zhang, X. et al. Irisin exhibits neuroprotection by preventing mitochondrial damage in Parkinson’s disease. NPJ Parkinsons Dis. 9, 13 (2023).
Zarbakhsh, S. et al. Irisin protects the substantia nigra dopaminergic neurons in the rat model of Parkinson’s disease. Iran. J. Basic Med Sci. 22, 722–728 (2019).
Kam, T. I. et al. Amelioration of pathologic alpha-synuclein-induced Parkinson’s disease by irisin. Proc. Natl Acad. Sci. USA 119, e2204835119 (2022).
Shi, X. et al. Relationship of irisin with disease severity and dopamine uptake in Parkinson’s disease patients. Neuroimage Clin. 41, 103555 (2024).
Jandova, T. et al. Long-Term Effect of Exercise on Irisin Blood Levels-Systematic Review and Meta-Analysis. Healthcare (Basel) 9, https://doi.org/10.3390/healthcare9111438 (2021).
Albrecht, E. et al. Irisin: Still chasing shadows. Mol. Metab. 34, 124–135 (2020).
Townsend, L. K., MacPherson, R. E. K. & Wright, D. C. New Horizon: Exercise and a Focus on Tissue-Brain Crosstalk. J. Clin. Endocrinol. Metab. 106, 2147–2163 (2021).
Cao, J., Zhou, A., Zhou, Z., Liu, H. & Jia, S. The role of GPLD1 in chronic diseases. J. Cell Physiol. 238, 1407–1415 (2023).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020).
Zhang, J. et al. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 10, 8315–8342 (2020).
Shen, Y., Wu, Q., Shi, J. & Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 132, 110928 (2020).
He, L., Wang, J., Yang, Y., Li, J. & Tu, H. Mitochondrial Sirtuins in Parkinson’s Disease. Neurochem Res 47, 1491–1502 (2022).
Dhiman, S., Mannan, A., Taneja, A., Mohan, M. & Singh, T. G. Sirtuin dysregulation in Parkinson’s disease: Implications of acetylation and deacetylation processes. Life Sci. 342, 122537 (2024).
Rose, G. et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp. Gerontol. 38, 1065–1070 (2003).
Bellizzi, D. et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85, 258–263 (2005).
He, X., Zeng, H. & Chen, J. X. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J. Cell Physiol. 234, 2252–2265 (2019).
Trinh, D. et al. Parkinson’s disease pathology is directly correlated to SIRT3 in human subjects and animal models: Implications for AAV.SIRT3-myc as a disease-modifying therapy. Neurobiol. Dis. 187, 106287 (2023).
Jiang, D. Q. et al. Microglia activation induces oxidative injury and decreases SIRT3 expression in dopaminergic neuronal cells. J. Neural Transm. (Vienna) 126, 559–568 (2019).
Starkov, A. A. et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 24, 7779–7788 (2004).
Leite, J. A., Ghirotto, B., Targhetta, V. P., de Lima, J. & Camara, N. O. S. Sirtuins as pharmacological targets in neurodegenerative and neuropsychiatric disorders. Br. J. Pharm. 179, 1496–1511 (2022).
Park, J. H. et al. Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol. Neurodegener. 15, 5 (2020).
Zhou, L., Pinho, R., Gu, Y. & Radak, Z. The Role of SIRT3 in Exercise and Aging. Cells 11, https://doi.org/10.3390/cells11162596 (2022).
Johnson, M. L. et al. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. A Biol. Sci. Med Sci. 70, 1386–1393 (2015).
Vargas-Ortiz, K. et al. Aerobic Training Increases Expression Levels of SIRT3 and PGC-1alpha in Skeletal Muscle of Overweight Adolescents Without Change in Caloric Intake. Pediatr. Exerc Sci. 27, 177–184 (2015).
Lanza, I. R. et al. Endurance exercise as a countermeasure for aging. Diabetes 57, 2933–2942 (2008).
Lee, S. et al. Physiological significance of elevated levels of lactate by exercise training in the brain and body. J. Biosci. Bioeng. 135, 167–175 (2023).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Wu, A., Lee, D. & Xiong, W. C. Lactate Metabolism, Signaling, and Function in Brain Development, Synaptic Plasticity, Angiogenesis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms241713398 (2023).
Hall, M. M., Rajasekaran, S., Thomsen, T. W. & Peterson, A. R. Lactate: Friend or Foe. PM R. 8, S8–S15 (2016).
Liguori, C. et al. Biomarkers of Cerebral Glucose Metabolism and Neurodegeneration in Parkinson’s Disease: A Cerebrospinal Fluid-Based Study. J. Parkinsons Dis. 12, 537–544 (2022).
Miyaue, N., Yabe, H. & Nagai, M. Serum growth differentiation factor 15, but not lactate, is elevated in patients with Parkinson’s disease. J. Neurol. Sci. 409, 116616 (2020).
Rivera-Brown, A. M. & Frontera, W. R. Principles of exercise physiology: responses to acute exercise and long-term adaptations to training. PM R. 4, 797–804 (2012).
Di Martino, S. et al. Aerobic rehabilitation program for improving muscle function in Parkinson’s disease. Restor. Neurol. Neurosci. 36, 13–20 (2018).
Cerexhe, L., Easton, C., Macdonald, E., Renfrew, L. & Sculthorpe, N. Blood lactate concentrations during rest and exercise in people with Multiple Sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 57, 103454 (2022).
Zhao, T. et al. Effect of Chronic Exercise Training on Blood Lactate Metabolism Among Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front Physiol. 12, 652023 (2021).
Terkelsen, M. H. et al. Neuroinflammation and Immune Changes in Prodromal Parkinson’s Disease and Other Synucleinopathies. J. Parkinsons Dis. 12, S149–S163 (2022).
Schrag, A. et al. Widening the Spectrum of Risk Factors, Comorbidities, and Prodromal Features of Parkinson Disease. JAMA Neurol. 80, 161–171 (2023).
Qu, Y. et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Parkinsons Dis. 9, 18 (2023).
Cerqueira, E., Marinho, D. A., Neiva, H. P. & Lourenco, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front Physiol. 10, 1550 (2019).
Papagianni, G. et al. The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: A systematic review and meta-analysis. Cytokine 164, 156157 (2023).
Hejazi, K., Mohammad Rahimi, G. R. & Rosenkranz, S. K. Effects of Exercise Training on Inflammatory and Cardiometabolic Risk Biomarkers in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Res Nurs. 25, 250–266 (2023).
Hejazi, K. & Wong, A. Effects of exercise training on inflammatory and cardiometabolic health markers in overweight and obese adults: a systematic review and meta-analysis of randomized controlled trials. J. Sports Med Phys. Fit. 63, 345–359 (2023).
Khalafi, M. et al. Influence of different modes of exercise training on inflammatory markers in older adults with and without chronic diseases: A systematic review and meta-analysis. Cytokine 169, 156303 (2023).
Zoladz, J. A. et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J. Physiol. Pharm. 65, 441–448 (2014).
Malczynska-Sims, P., Chalimoniuk, M., Wronski, Z., Marusiak, J. & Sulek, A. High-intensity interval training modulates inflammatory response in Parkinson’s disease. Aging Clin. Exp. Res 34, 2165–2176 (2022).
de Almeida, E. J. R., Ibrahim, H. J., Chitolina Schetinger, M. R., de Andrade, C. M. & Cardoso, A. M. Modulation of Inflammatory Mediators and Microglial Activation Through Physical Exercise in Alzheimer’s and Parkinson’s Diseases. Neurochem Res 47, 3221–3240 (2022).
Mogi, M. et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180, 147–150 (1994).
Boka, G. et al. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 172, 151–154 (1994).
Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673 (2022).
Eidson, L. N. et al. Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflammation 14, 164 (2017).
Reale, M. et al. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 23, 55–63 (2009).
Brodacki, B. et al. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 441, 158–162 (2008).
Mogi, M. et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 165, 208–210 (1994).
Blum-Degen, D. et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 202, 17–20 (1995).
Chen, H. et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 60, 1059–1064 (2003).
Aloe, L. & Fiore, M. TNF-alpha expressed in the brain of transgenic mice lowers central tyroxine hydroxylase immunoreactivity and alters grooming behavior. Neurosci. Lett. 238, 65–68 (1997).
Peter, I. et al. Anti-Tumor Necrosis Factor Therapy and Incidence of Parkinson Disease Among Patients With Inflammatory Bowel Disease. JAMA Neurol. 75, 939–946 (2018).
Zhu, Z., Gao, Z. & Li, K. Controversy of Preoperative Exposure to Tumor Necrosis Factor Inhibitors in Surgical and Infectious Complications of Inflammatory Bowel Disease. Gastroenterology 164, 307–308 (2023).
Zheng, G. et al. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Aging Neurosci. 11, 98 (2019).
Scalzo, P., Kummer, A., Cardoso, F. & Teixeira, A. L. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci. Lett. 468, 56–58 (2010).
Diaz, K., Kohut, M. L., Russell, D. W. & Stegemoller, E. L. Peripheral inflammatory cytokines and motor symptoms in persons with Parkinson’s disease. Brain Behav. Immun. Health 21, 100442 (2022).
Muller, T., Blum-Degen, D., Przuntek, H. & Kuhn, W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurol. Scand. 98, 142–144 (1998).
Selikhova, M. V., Kushlinskii, N. E., Lyubimova, N. V. & Gusev, E. I. Impaired production of plasma interleukin-6 in patients with Parkinson’s disease. Bull. Exp. Biol. Med 133, 81–83 (2002).
Rose-John, S., Jenkins, B. J., Garbers, C., Moll, J. M. & Scheller, J. Targeting IL-6 trans-signalling: past, present and future prospects. Nat. Rev. Immunol. 23, 666–681 (2023).
Gruol, D. L. & Nelson, T. E. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 15, 307–339 (1997).
de Baat, A., Trinh, B., Ellingsgaard, H. & Donath, M. Y. Physiological role of cytokines in the regulation of mammalian metabolism. Trends Immunol. 44, 613–627 (2023).
Mehta, N., Luthra, N. S., Corcos, D. M. & Fantuzzi, G. C-reactive protein as the biomarker of choice to monitor the effects of exercise on inflammation in Parkinson’s disease. Front Immunol. 14, 1178448 (2023).
Qiu, X. et al. C-Reactive Protein and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Neurol. 10, 384 (2019).
Sawada, H. et al. Baseline C-Reactive Protein Levels and Life Prognosis in Parkinson Disease. PLoS One 10, e0134118 (2015).
Andican, G. et al. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol. Belg. 112, 155–159 (2012).
Sanjari Moghaddam, H. et al. Cerebrospinal Fluid C-Reactive Protein in Parkinson’s Disease: Associations with Motor and Non-motor Symptoms. Neuromolecular Med 20, 376–385 (2018).
Lindqvist, D. et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease-associations with depression, fatigue, and cognitive impairment. Brain Behav. Immun. 33, 183–189 (2013).
Rose, G. L., Skinner, T. L., Mielke, G. I. & Schaumberg, M. A. The effect of exercise intensity on chronic inflammation: A systematic review and meta-analysis. J. Sci. Med Sport 24, 345–351 (2021).
Nalls, M. A. et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46, 989–993 (2014).
Yuan, Z. et al. Research progress of NLRP3 inflammasome and its inhibitors with aging diseases. Eur. J. Pharm. 957, 175931 (2023).
Del Rosso, S. et al. Long-term effects of different exercise training modes on cytokines and adipokines in individuals with overweight/obesity and cardiometabolic diseases: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. Obes. Rev. 24, e13564 (2023).
Behboudi, L. & Eizadi, M. The Modifying Impact of Long-Term Endurance Training on Inflammatory Cytokine IL-1B Level and VO2max in Premenopausal Women with Abdominal Obesity. Jundishapur J. Chronic Dis. Care 6, e57180 (2017).
Subbarayan, M. S., Joly-Amado, A., Bickford, P. C. & Nash, K. R. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharm. Ther. 231, 107989 (2022).
Hatcher-Martin, J. M. et al. Cerebrospinal fluid biomarkers in Parkinson’s disease with freezing of gait: an exploratory analysis. NPJ Parkinsons Dis. 7, 105 (2021).
Kumar, P. et al. Physical exercise is associated with a reduction in plasma levels of fractalkine, TGF-beta1, eotaxin-1 and IL-6 in younger adults with mobility disability. PLoS One 17, e0263173 (2022).
Njerve, I. U. et al. Effects of long-term exercise training on adipose tissue expression of fractalkine and MCP-1 in patients with type 2 diabetes and stable coronary artery disease: a substudy of a randomized controlled trial. Diabetes Metab. Syndr. Obes. 9, 55–62 (2016).
Szymura, J., Kubica, J., Wiecek, M. & Pera, J. The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease. J. Clin. Med. 9, https://doi.org/10.3390/jcm9010184 (2020).
De Miguel, Z. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Yuste-Checa, P., Bracher, A. & Hartl, F. U. The chaperone Clusterin in neurodegeneration-friend or foe?. Bioessays 44, e2100287 (2022).
Filippini, A. et al. Extracellular clusterin limits the uptake of alpha-synuclein fibrils by murine and human astrocytes. Glia 69, 681–696 (2021).
Kitamura, Y. et al. Proteomic Profiling of Exosomal Proteins for Blood-based Biomarkers in Parkinson’s Disease. Neuroscience 392, 121–128 (2018).
De Pablo-Fernandez, E. et al. Neuroendocrine abnormalities in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88, 176–185 (2017).
Luthra, N. S., Christou, D. D., Clow, A. & Corcos, D. M. Targeting neuroendocrine abnormalities in Parkinson’s disease with exercise. Front Neurosci. 17, 1228444 (2023).
Sieurin, J. et al. Occupational stress and risk for Parkinson’s disease: A nationwide cohort study. Mov. Disord. 33, 1456–1464 (2018).
Vlajinac, H. et al. The stressful life events and Parkinson’s disease: a case-control study. Stress Health 29, 50–55 (2013).
Kalaitzakis, M. E., Gentleman, S. M. & Pearce, R. K. Disturbed sleep in Parkinson’s disease: anatomical and pathological correlates. Neuropathol. Appl Neurobiol. 39, 644–653 (2013).
Leng, Y. et al. Association of Circadian Abnormalities in Older Adults With an Increased Risk of Developing Parkinson Disease. JAMA Neurol. 77, 1270–1278 (2020).
Han, K., Kim, B., Lee, S. H. & Kim, M. K. A nationwide cohort study on diabetes severity and risk of Parkinson disease. NPJ Parkinsons Dis. 9, 11 (2023).
Aune, D. et al. Diabetes mellitus, prediabetes and the risk of Parkinson’s disease: a systematic review and meta-analysis of 15 cohort studies with 29.9 million participants and 86,345 cases. Eur. J. Epidemiol. 38, 591–604 (2023).
Knekt, P. et al. Serum vitamin D and the risk of Parkinson disease. Arch. Neurol. 67, 808–811 (2010).
Ross, G. W., Petrovitch, H. & Abbott, R. D. Serum vitamin D and risk of Parkinson’s disease. Mov. Disord. 31, 993–935 (2016).
Newmark, H. L. & Newmark, J. Vitamin D and Parkinson’s disease-a hypothesis. Mov. Disord. 22, 461–468 (2007).
Levy, G. The relationship of Parkinson disease with aging. Arch. Neurol. 64, 1242–1246 (2007).
Breen, D. P. et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 71, 589–595 (2014).
Hartmann, A., Veldhuis, J. D., Deuschle, M., Standhardt, H. & Heuser, I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 18, 285–289 (1997).
Costa, C. M. et al. Levels of cortisol and neurotrophic factor brain-derived in Parkinson’s disease. Neurosci. Lett. 708, 134359 (2019).
Skogar, O. et al. Diurnal salivary cortisol concentrations in Parkinson’s disease: increased total secretion and morning cortisol concentrations. Int J. Gen. Med 4, 561–569 (2011).
Djamshidian, A. et al. Salivary cortisol levels in Parkinson’s disease and its correlation to risk behaviour. J. Neurol. Neurosurg. Psychiatry 82, 1107–1111 (2011).
Sanchez-Gomez, A. et al. Peripheral insulin and amylin levels in Parkinson’s disease. Parkinsonism Relat. Disord. 79, 91–96 (2020).
Evatt, M. L. et al. High prevalence of hypovitaminosis D status in patients with early Parkinson disease. Arch. Neurol. 68, 314–319 (2011).
Evatt, M. L. et al. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch. Neurol. 65, 1348–1352 (2008).
Videnovic, A. et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 71, 463–469 (2014).
Zimmermann, M. et al. The longevity gene Klotho and its cerebrospinal fluid protein profiles as a modifier for Parkinson s disease. Eur. J. Neurol. 28, 1557–1565 (2021).
Kakar, R. S. et al. Peripheral Klotho and Parkinson’s Disease. Mov. Disord. 36, 1274–1276 (2021).
Sancesario, G. M. et al. Biofluids profile of alpha-Klotho in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 90, 62–64 (2021).
Beserra, A. H. N. et al. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatry Psychother. 40, 360–368 (2018).
Kim, H.-J. & Kim, D.-H. Effect of Different Exercise Intensity on Blood Melatonin Density in Sleep Disordered Rats. J. Korean Soc. Phys. Med. 0, 45–53 (2014).
Al-Rawaf, H. A., Gabr, S. A., Iqbal, A. & Alghadir, A. H. Effects of High-Intensity Interval Training on Melatonin Function and Cellular Lymphocyte Apoptosis in Sedentary Middle-Aged Men. Medicina (Kaunas) 59, https://doi.org/10.3390/medicina59071201 (2023).
Borghouts, L. B. & Keizer, H. A. Exercise and insulin sensitivity: a review. Int J. Sports Med 21, 1–12 (2000).
Sampath Kumar, A. et al. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med 62, 98–103 (2019).
Zhang, J. & Cao, Z. B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 14, https://doi.org/10.3390/nu14132652 (2022).
Amaro-Gahete, F. J. et al. Exercise training increases the S-Klotho plasma levels in sedentary middle-aged adults: A randomised controlled trial. The FIT-AGEING study. J. Sports Sci. 37, 2175–2183 (2019).
Matsubara, T. et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 306, H348–H355 (2014).
Correa, H. L. et al. A systematic review and meta-analysis demonstrating Klotho as an emerging exerkine. Sci. Rep. 12, 17587 (2022).
Viho, E. M. G. et al. Corticosteroid Action in the Brain: The Potential of Selective Receptor Modulation. Neuroendocrinology 109, 266–276 (2019).
van Wamelen, D. J., Wan, Y. M., Ray Chaudhuri, K. & Jenner, P. Stress and cortisol in Parkinson’s disease. Int Rev. Neurobiol. 152, 131–156 (2020).
Smith, L. K., Jadavji, N. M., Colwell, K. L., Katrina Perehudoff, S. & Metz, G. A. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. Eur. J. Neurosci. 27, 2133–2146 (2008).
Seifried, C. et al. Diurnal variation of hypothalamic function and chronic subthalamic nucleus stimulation in Parkinson’s disease. Neuroendocrinology 97, 283–290 (2013).
van den Heuvel, L. L. et al. Hair glucocorticoid levels in Parkinson’s disease. Psychoneuroendocrinology 117, 104704 (2020).
Haglin, L. & Backman, L. Covariation between plasma phosphate and daytime cortisol in early Parkinson’s disease. Brain Behav. 6, e00556 (2016).
Muller, T., Welnic, J. & Muhlack, S. Acute levodopa administration reduces cortisol release in patients with Parkinson’s disease. J. Neural Transm. (Vienna) 114, 347–350 (2007).
Hackney, A. C. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev. Endocrinol. Metab. 1, 783–792 (2006).
Smyth, N. et al. Endurance exercise reduces cortisol in Parkinson’s disease with mild cognitive impairment. Mov. Disord. 34, 1238–1239 (2019).
Chen, W., Cai, W., Hoover, B. & Kahn, C. R. Insulin action in the brain: cell types, circuits, and diseases. Trends Neurosci. 45, 384–400 (2022).
Moroo, I. et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol. 87, 343–348 (1994).
Takahashi, M. et al. Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 204, 201–204 (1996).
Sekar, S. & Taghibiglou, C. Elevated nuclear phosphatase and tensin homolog (PTEN) and altered insulin signaling in substantia nigral region of patients with Parkinson’s disease. Neurosci. Lett. 666, 139–143 (2018).
Hogg, E. et al. High Prevalence of Undiagnosed Insulin Resistance in Non-Diabetic Subjects with Parkinson’s Disease. J. Parkinsons Dis. 8, 259–265 (2018).
Jones, K. T. et al. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J. Neurochem 140, 728–740 (2017).
Baladi, M. G., Horton, R. E., Owens, W. A., Daws, L. C. & France, C. P. Eating high fat chow decreases dopamine clearance in adolescent and adult male rats but selectively enhances the locomotor stimulating effects of cocaine in adolescents. Int J. Neuropsychopharmacol. 18, pyv024 (2015).
Stouffer, M. A. et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun. 6, 8543 (2015).
Hong, C. T. et al. Insulin Resistance Promotes Parkinson’s Disease through Aberrant Expression of alpha-Synuclein, Mitochondrial Dysfunction, and Deregulation of the Polo-Like Kinase 2 Signaling. Cells 9, https://doi.org/10.3390/cells9030740 (2020).
Kao, S. Y. Rescue of alpha-synuclein cytotoxicity by insulin-like growth factors. Biochem Biophys. Res Commun. 385, 434–438 (2009).
Cullinane, P. W. et al. Type 2 Diabetes and Parkinson’s Disease: A Focused Review of Current Concepts. Mov. Disord. https://doi.org/10.1002/mds.29298 (2022).
Kalinderi, K., Papaliagkas, V. & Fidani, L. GLP-1 Receptor Agonists: A New Treatment in Parkinson’s Disease. Int J Mol Sci 25, https://doi.org/10.3390/ijms25073812 (2024).
Meissner, W. G. et al. Trial of Lixisenatide in Early Parkinson’s Disease. N. Engl. J. Med 390, 1176–1185 (2024).
Mann, S. et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab. Res Rev. 30, 257–268 (2014).
Park, S., Jang, J. S., Jun, D. W. & Hong, S. M. Exercise enhances insulin and leptin signaling in the cerebral cortex and hypothalamus during dexamethasone-induced stress in diabetic rats. Neuroendocrinology 82, 282–293 (2005).
Muller, A. P. et al. Exercise increases insulin signaling in the hippocampus: physiological effects and pharmacological impact of intracerebroventricular insulin administration in mice. Hippocampus 21, 1082–1092 (2011).
Jeong, J. H., Koo, J. H., Cho, J. Y. & Kang, E. B. Neuroprotective effect of treadmill exercise against blunted brain insulin signaling, NADPH oxidase, and Tau hyperphosphorylation in rats fed a high-fat diet. Brain Res Bull. 142, 374–383 (2018).
Kullmann, S. et al. Exercise restores brain insulin sensitivity in sedentary adults who are overweight and obese. JCI Insight 7, https://doi.org/10.1172/jci.insight.161498 (2022).
Duzel, E., van Praag, H. & Sendtner, M. Can physical exercise in old age improve memory and hippocampal function. Brain 139, 662–673 (2016).
Schwarz, A. J., Brasel, J. A., Hintz, R. L., Mohan, S. & Cooper, D. M. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 81, 3492–3497 (1996).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Zeldich, E. et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J. Biol. Chem. 289, 24700–24715 (2014).
Yamazaki, Y. et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys. Res Commun. 398, 513–518 (2010).
Semba, R. D. et al. Plasma klotho and mortality risk in older community-dwelling adults. J. Gerontol. A Biol. Sci. Med Sci. 66, 794–800 (2011).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Imura, A. et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 565, 143–147 (2004).
Chang, Q. et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 (2005).
Leon, J. et al. Peripheral Elevation of a Klotho Fragment Enhances Brain Function and Resilience in Young, Aging, and alpha-Synuclein Transgenic Mice. Cell Rep. 20, 1360–1371 (2017).
Baluchnejadmojarad, T. et al. The anti-aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6-hydroxydopamine rat model of Parkinson’s disease: Involvement of PKA/CaMKII/CREB signaling. Exp. Gerontol. 100, 70–76 (2017).
Dubal, D. B. et al. Life extension factor klotho enhances cognition. Cell Rep. 7, 1065–1076 (2014).
Yokoyama, J. S. et al. Variation in longevity gene KLOTHO is associated with greater cortical volumes. Ann. Clin. Transl. Neurol. 2, 215–230 (2015).
Driscoll, I. F. et al. KLOTHO KL-VS heterozygosity is associated with diminished age-related neuroinflammation, neurodegeneration, and synaptic dysfunction in older cognitively unimpaired adults. Alzheimers Dement 20, 5347–5356 (2024).
Belloy, M. E. et al. Association of Klotho-VS Heterozygosity With Risk of Alzheimer Disease in Individuals Who Carry APOE4. JAMA Neurol. 77, 849–862 (2020).
Kundu, P. et al. Serum Levels of alpha-Klotho Are Correlated with Cerebrospinal Fluid Levels and Predict Measures of Cognitive Function. J. Alzheimers Dis. 86, 1471–1481 (2022).
Zimmermann, M. et al. Association of elevated cerebrospinal fluid levels of the longevity protein alpha-Klotho with a delayed onset of cognitive impairment in Parkinson’s disease patients. Eur. J. Neurol., e16388, https://doi.org/10.1111/ene.16388 (2024).
Semba, R. D. et al. Low Plasma Klotho Concentrations and Decline of Knee Strength in Older Adults. J. Gerontol. A Biol. Sci. Med Sci. 71, 103–108 (2016).
Crasto, C. L. et al. Relationship of low-circulating “anti-aging” klotho hormone with disability in activities of daily living among older community-dwelling adults. Rejuvenation Res 15, 295–301 (2012).
Saghiv, M. S., Sira, D. B., Goldhammer, E. & Sagiv, M. The effects of aerobic and anaerobic exercises on circulating soluble-Klotho and IGF-I in young and elderly adults and in CAD patients. J. Circ. Biomark. 6, 1849454417733388 (2017).
Amaro-Gahete, F. J. et al. Association of physical activity and fitness with S-Klotho plasma levels in middle-aged sedentary adults: The FIT-AGEING study. Maturitas 123, 25–31 (2019).
Jin, Z., Zhang, Z., Ke, J., Wang, Y. & Wu, H. Exercise-Linked Irisin Prevents Mortality and Enhances Cognition in a Mice Model of Cerebral Ischemia by Regulating Klotho Expression. Oxid. Med Cell Longev. 2021, 1697070 (2021).
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Patel, D. & Bordoni, B. in StatPearls (2025).
Calabresi, P. et al. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 14, 176 (2023).
Polymeropoulos, M. H. et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047 (1997).
Singleton, A. B. et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841 (2003).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Goldman, J. G. et al. Cerebrospinal fluid, plasma, and saliva in the BioFIND study: Relationships among biomarkers and Parkinson’s disease Features. Mov. Disord. 33, 282–288 (2018).
Fairfoul, G. et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 3, 812–818 (2016).
Groveman, B. R. et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol. Commun. 6, 7 (2018).
Garrido, A. et al. alpha-synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease. Ann. Clin. Transl. Neurol. 6, 1024–1032 (2019).
Kang, U. J. et al. Comparative study of cerebrospinal fluid alpha-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 34, 536–544 (2019).
van Rumund, A. et al. alpha-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann. Neurol. 85, 777–781 (2019).
Orru, C. D. et al. A rapid alpha-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy. Ann. Clin. Transl. Neurol. n/a, https://doi.org/10.1002/acn3.51280 (2020).
Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 140, 49–62 (2020).
Bargar, C. et al. Discrimination of MSA-P and MSA-C by RT-QuIC analysis of olfactory mucosa: the first assessment of assay reproducibility between two specialized laboratories. Mol. Neurodegener. 16, 82 (2021).
Majbour, N. K. et al. Cerebrospinal alpha-Synuclein Oligomers Reflect Disease Motor Severity in DeNoPa Longitudinal Cohort. Mov. Disord. 36, 2048–2056 (2021).
Russo, M. J. et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 9, 179 (2021).
Poggiolini, I. et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain 145, 584–595 (2022).
Grossauer, A. et al. alpha-Synuclein Seed Amplification Assays in the Diagnosis of Synucleinopathies Using Cerebrospinal Fluid-A Systematic Review and Meta-Analysis. Mov. Disord. Clin. Pr. 10, 737–747 (2023).
Yoo, D. et al. Diagnostic value of alpha-synuclein seeding amplification assays in alpha-synucleinopathies: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 104, 99–109 (2022).
Wang, Z. et al. Skin alpha-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.3311 (2020).
Mammana, A. et al. RT-QuIC Detection of Pathological α-Synuclein in Skin Punches of Patients with Lewy Body Disease. Mov. Disord. 36, 2173–2177 (2021).
Kuzkina, A. et al. Diagnostic value of skin RT-QuIC in Parkinson’s disease: a two-laboratory study. npj Parkinson’s Dis. 7, 99 (2021).
De Luca, C. M. G. et al. Efficient RT-QuIC seeding activity for alpha-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl. neurodegeneration 8, 24 (2019).
Vivacqua, G. et al. Salivary alpha-Synuclein RT-QuIC Correlates with Disease Severity in de novo Parkinson’s Disease. Mov. Disord. https://doi.org/10.1002/mds.29246 (2022).
Luan, M. et al. Diagnostic Value of Salivary Real-Time Quaking-Induced Conversion in Parkinson’s Disease and Multiple System Atrophy. Movement Disord. n/a, https://doi.org/10.1002/mds.28976 (2022).
Kluge, A. et al. Detection of neuron-derived pathological alpha-synuclein in blood. Brain 145, 3058–3071 (2022).
Okuzumi, A. et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat. Med. https://doi.org/10.1038/s41591-023-02358-9 (2023).
Liu, W. et al. Regular Aerobic Exercise-Alleviated Dysregulation of CAMKIIalpha Carbonylation to Mitigate Parkinsonism via Homeostasis of Apoptosis With Autophagy. J. Neuropathol. Exp. Neurol. 79, 46–61 (2020).
Hwang, D. J. et al. Neuroprotective effect of treadmill exercise possibly via regulation of lysosomal degradation molecules in mice with pharmacologically induced Parkinson’s disease. J. Physiol. Sci. 68, 707–716 (2018).
Jang, Y. C. et al. Association of exercise-induced autophagy upregulation and apoptosis suppression with neuroprotection against pharmacologically induced Parkinson’s disease. J. Exerc Nutr. Biochem 22, 1–8 (2018).
Almeida, M. F. et al. Effects of mild running on substantia nigra during early neurodegeneration. J. Sports Sci. 36, 1363–1370 (2018).
Koo, J. H. & Cho, J. Y. Treadmill Exercise Attenuates alpha-Synuclein Levels by Promoting Mitochondrial Function and Autophagy Possibly via SIRT1 in the Chronic MPTP/P-Induced Mouse Model of Parkinson’s Disease. Neurotox. Res 32, 473–486 (2017).
Mollenhauer, B. et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov. Disord. 35, 1999–2008 (2020).
Buhmann, C., Magnus, T. & Choe, C. U. Blood neurofilament light chain in Parkinson’s disease. J. Neural Transm. (Vienna) 130, 755–762 (2023).
Alagaratnam, J. et al. Correlation between CSF and blood neurofilament light chain protein: a systematic review and meta-analysis. BMJ Neurol. Open 3, e000143 (2021).
Ashton, N. J. et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 12, 3400 (2021).
Ercan, Z., Bilek, F. & Demir, C. F. The effect of aerobic exercise on Neurofilament light chain and glial Fibrillary acidic protein level in patients with relapsing remitting type multiple sclerosis. Mult. Scler. Relat. Disord. 55, 103219 (2021).
Frederiksen, K. S. et al. Aerobic exercise does not affect serum neurofilament light in patients with mild Alzheimer’s disease. Front. Neurosci. 17, https://doi.org/10.3389/fnins.2023.1108191 (2023).
Kasanga, E. A. et al. Moderate intensity aerobic exercise in 6-OHDA-lesioned rats alleviates established motor deficits and reduces neurofilament light and glial fibrillary acidic protein serum levels without increased striatal dopamine or tyrosine hydroxylase protein. bioRxiv. 2023.2007.2011.548638, https://doi.org/10.1101/2023.07.11.548638 (2023).
Hill, M. A. & Gammie, S. C. Alzheimer’s disease large-scale gene expression portrait identifies exercise as the top theoretical treatment. Sci. Rep. 12, 17189 (2022).
Paillard, T., Blain, H. & Bernard, P. L. The impact of exercise on Alzheimer’s disease progression. Expert Rev. Neurother. 24, 333–342 (2024).
Flores, V. A. et al. Effects of aerobic, resistance, and combined exercise training on health-related quality of life in multiple sclerosis: Systematic review and meta-analysis. Mult. Scler. Relat. Disord. 75, 104746 (2023).
Mogi, M. et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 270, 45–48 (1999).
Lippi, G., Mattiuzzi, C. & Sanchis-Gomar, F. Updated overview on interplay between physical exercise, neurotrophins, and cognitive function in humans. J. Sport Health Sci. 9, 74–81 (2020).
Schroder, J. B. et al. Immune Cell Activation in the Cerebrospinal Fluid of Patients With Parkinson’s Disease. Front Neurol. 9, 1081 (2018).
Li, Y. et al. Parkinson’s disease peripheral immune biomarker profile: a multicentre, cross-sectional and longitudinal study. J. Neuroinflammation 19, 116 (2022).
Troseid, M. et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur. Heart J. 25, 349–355 (2004).
Clifford, B. K., Kaakoush, N. O., Tedla, N., Goldstein, D. & Simar, D. The effect of exercise intensity on the inflammatory profile of cancer survivors: A randomised crossover study. Eur. J. Clin. Invest 53, e13984 (2023).
Bagheri, V., Khorramdelazad, H., Hassanshahi, G., Moghadam-Ahmadi, A. & Vakilian, A. CXCL12 and CXCR4 in the Peripheral Blood of Patients with Parkinson’s Disease. Neuroimmunomodulation 25, 201–205 (2018).
Tsukui, S. et al. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J. Obes. Relat. Metab. Disord. 24, 1207–1211 (2000).
Choe, C. U. et al. Subclinical Cardiac Microdamage, Motor Severity, and Cognition in Parkinson’s Disease. Mov. Disord. 35, 1863–1868 (2020).
Bordbar, S., Bigi, M. A., Aslani, A., Rahimi, E. & Ahmadi, N. Effect of endurance and strength exercise on release of brain natriuretic peptide. J. Cardiovasc Dis. Res 3, 22–25 (2012).
Malandish, A., Ghadamyari, N., Karimi, A. & Naderi, M. The role of exercise training on cardiovascular peptides in patients with heart failure: A systematic review and meta-analysis. Curr. Res Physiol. 5, 270–286 (2022).
Conroy, S. M. et al. Impact of aerobic exercise on levels of IL-4 and IL-10: results from two randomized intervention trials. Cancer Med 5, 2385–2397 (2016).
Kouti, L. et al. Nitric oxide and peroxynitrite serum levels in Parkinson’s disease: correlation of oxidative stress and the severity of the disease. Eur. Rev. Med Pharm. Sci. 17, 964–970 (2013).
Santos-Lobato, B. L. et al. Levodopa-induced dyskinesias in Parkinson’s disease increase cerebrospinal fluid nitric oxide metabolites’ levels. J. Neural Transm. (Vienna) 129, 55–63 (2022).
Arefirad, T. et al. Effect of exercise training on nitric oxide and nitrate/nitrite (NOx) production: A systematic review and meta-analysis. Front Physiol. 13, 953912 (2022).
Kang, J. H. et al. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s Progression Markers Initiative study. Acta Neuropathol. 131, 935–949 (2016).
Stillwell, S. B., Fineout-Overholt, E., Melnyk, B. M. & Williamson, K. M. Evidence-based practice, step by step: searching for the evidence. Am. J. Nurs. 110, 41–47 (2010).
Acknowledgements
The work was supported by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (Award Number K23NS123506 and U01NS113851). Work was also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422, as well as the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (Award number T32-HD101395 and T32-HD07418). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Concept and design—N.L., U.K., and D.M.C.; original draft preparation—N.L., N.M., M.M., G.F., U.K., G.L., J.H., D.M.C.; editing— N.L., N.M., M.M., G.C., G.F., U.K., G.L., N.F., J.H., M.T., D.M.C. Final revised version: all co-authors read and approved the completed version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luthra, N.S., Mehta, N., Munoz, M.J. et al. Aerobic exercise-induced changes in fluid biomarkers in Parkinson’s disease. npj Parkinsons Dis. 11, 190 (2025). https://doi.org/10.1038/s41531-025-01042-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01042-8
This article is cited by
-
Voluntary exercise increases striatal dopamine release and improves motor performance in aging mice
npj Parkinson's Disease (2025)