Abstract
Recent work has shown that REM density, defined as the number of rapid eye movements per REM sleep minute, is decreased in people with Parkinson’s disease (PD) and is associated with greater bradykinesia, suggesting a motor component. This study explored the association of REM density with gait, cognitive function, and heart rate variability (HRV). Seventy-seven PD patients underwent overnight polysomnography. Gait was evaluated using wearable technology during preferred-speed walking and while walking with a cognitive task (dual tasking). Lower REM density was associated with poorer gait and mobility, particularly during dual-tasking and turning, but not with HRV. Patients taking evening slow-release dopaminergic medication showed higher REM density compared to drug-naïve patients or those on daytime medication. These findings highlight the role of motor and cognitive functions in the generation of rapid eye movements in PD. Administration of evening medications appears to improve REM density, likely by alleviating motor control deficits.
Similar content being viewed by others
Introduction
Sleep is generally categorized into rapid eye movement (REM) sleep and non-REM sleep1. REM sleep is characterized by rapid eye movements (REMs), neural activity resembling wakefulness, and skeletal paralysis. REM sleep was deemed essential to memory consolidation and emotional regulation, reflecting cognitive components2,3. REM sleep also encompasses fluctuations of sympathetic activity with mild changes in parasympathetic activity4. REMs, the involuntary oculomotor movements that define REM sleep, are generated by cortical and subcortical regions5, likely involving both autonomic and motor components, specifically the ponto-geniculo-occipital (PGO) waves6, with the pontine tegmentum and the pontine reticular formation playing a central role7. Furthermore, REMs, a phasic REM sleep activity, have been associated with activation of the motor cortex during sleep8. These regions overlap significantly with structures vulnerable to degeneration in PD, including the pedunculopontine nucleus (PPN) and components of the reticular activating system, which are involved in both arousal regulation and motor control.
The speed and rhythm of REMs have been evaluated by calculating REM density, defined as the number of REMs per minute during REM sleep9,10. REM density increases across the night with a progressive reduction in sleep pressure, which refers to the increasing need for sleep that builds during wakefulness and dissipates during sleep9. This inverse relationship, along with reduced REM density following sleep deprivation, suggests that REM density may reflect a balance between satisfying sleep needs and the build-up of arousal pressure9,11. REM density, like REM sleep, was also associated with cognitive performance12,13 and may have a putative role in emotional memory consolidation14. Increased REM density has been observed in patients with post-traumatic stress disorder (PTSD) and major depression (MD)7, both of which are associated with hyperarousal15. Interestingly, reduced REM density was observed in people with Parkinson’s disease (PD)16,17, a disease associated with hypokinesia and hypoarousal, suggesting that dysfunctions in neurotransmitters involved in arousal regulation may underlie these alterations and are likely, not unidirectional9.
The clinical manifestation of PD is multifaceted, affecting both the motor and non-motor aspects of daily living, including sleep disorders, cognitive impairments, and autonomic dysfunction18,19. Recent work has shown that REM density is associated with motor impairment in PD, specifically with the bradykinesia subscore of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III)16. From a mechanistic perspective, the neurodegenerative process may lead to bradykinesia and increased variability of motor movement20,21, potentially disrupting both the pace and rhythm of REMs and other movements. Both eye movements and gait are bilaterally coupled motions that can be significantly affected in PD21. Among gait features, bradykinesia is most closely linked to gait speed and step length, stemming from the pace domain21. As PD progresses, gait becomes less automatic and more dysrhythmic, producing higher variability of movement and requiring increased cognitive resources21. As the task becomes more complex, such as during dual-task or turning situations, the reliance on limited motor and cognitive resources may reveal a motor-cognitive interference21,22, resulting in the deterioration of one or both tasks. Interestingly, while PD is associated with increased gait variability, autonomic dysfunction in PD patients is often evident in reduced heart rate variability (HRV)23,24, reflecting impaired modulation of the heart’s intrinsic rhythmicity by autonomic inputs rather than increased dysrhythmicity. While gait rhythm is actively generated by central nervous system circuits, including cortical, basal ganglia, and brainstem structures, heart rhythm is intrinsically generated by cardiac pacemaker cells and modulated by autonomic influences. Thus, although both gait and HRV are affected by neurodegenerative processes, their distinct physiological organization may differentially affect variability. The extent to which these motor and autonomic changes contribute to REM density regulation remains unclear.
The relationship between the neurodegenerative process and reduced REM density raises another question relating to dopaminergic medication. Generally, administering dopaminergic medication before bedtime, especially in controlled release (CR) formulations, may improve nocturnal hypokinesia25 and enhance overnight sleep quality26. However, whether the improved motor functioning resulting from dopaminergic medication affects REM density is unknown.
This study aimed to explore the relationship between the motor, cognitive, and autonomic systems and REM density in patients with PD. We speculated that lower REM density would be associated with poorer gait metrics from the pace domain, but also with HRV. Given that REM density was previously associated with cognitive function, we further speculated that lower REM density will be associated with worse cognitive function as well as gait measures under complex walking situations, underlining motor-cognitive impairment. Lastly, we speculated that patients receiving evening medication would exhibit higher REM density (better) than drug-naïve patients or those receiving medication only during the day.
Results
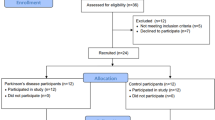
A total of 77 PD patients met the inclusion criteria for this study. Three patients with extremely high REM density values were excluded from all subsequent analyses, resulting in a final sample of 74 patients (mean age 66.07 ± 8.23 years, disease duration 3.54 ± 3.71 years). No outliers were identified at the lower end of the distribution. Descriptive statistics for demographics and sleep measures are presented in Table 1. None of the patients used sleep medication on the night of the PSG. However, four patients regularly used hypnotics: two used melatonin, one used Zopiclone, and one used Zolpidem (non-benzodiazepine hypnotics). Based on the PSG assessment, 31 patients (41.89%) were diagnosed with RBD. No significant difference in REM density was observed between the PD RBD+ group (5.04 ± 3.02) and the PD RBD– group (4.84 ± 2.79, p = 0.852), nor between males and females (p = 0.673). REM density was also not associated with RSWA% or RBDQ scores (p = 0.627 and p = 0.577, respectively). Eight patients using selective serotonin reuptake inhibitors had comparable REM density values (3.91 ± 2.43) to the 66 patients not receiving SSRI treatment (5.05 ± 2.91, p = 0.231). The individual data (including outliers) containing demographic, clinical, REM density, and medication characteristics are presented in Supplementary Table 1.
Motor, cognitive, and autonomic functions and REM density
Figure 1 presents a visual representation of 15 s of EOG during REM sleep, diurnal gait data, and ECG during REM. Gait data were acquired for 67 patients (Table 2); data from 7 participants were missing due to technical issues or time constraints during data collection. Correlation heatmaps of REM density and REMs interval and variability measures with gait and HRV variables, adjusted for age, are shown in Fig. 2. We observed a negative association between REM density and bradykinesia scores (Fig. 2). No significant correlations were found between REM density and rigidity or tremor scores (p > 0.334), nor with demographic or other sleep variables.
A, B Left and right EOG channels during REM sleep. C ECG data recorded during REM sleep from the same patient. D Vertical acceleration signal from the lumbar sensor during diurnal gait bout. Rapid eye movements (REMs) were detected from EOG channels and were used to calculate REM density, defined as the number of REMs divided by total REM duration in minutes. For HRV, a single, artifact-free five-minute PSG segment was selected and analysed for each participant. The horizontal arrows indicate time intervals between REMs (A), subsequent heartbeats (C), and subsequent steps (D). A stride consists of subsequent left and right steps. Two components were extracted across the three domains: Rhythm – mean REMs interval, stride mean time and mean normal-to-normal heartbeat interval (NN). Variability – REMs interval std and REMs interval coefficient of variation (CV), stride time CV, and heartbeat interval std (SDNN).
Gait variables are categorized into three domains: pace, rhythm, and variability. Correlation strength is indicated by color, with blue representing negative correlations and red representing positive correlations. Significant associations at p < 0.05 are annotated with their corresponding r-values. Analyses were based on different sets of available participant data: REMs, clinical, and cognitive measures (n = 74); HRV (n = 52); and gait (n = 67). See Table 2 for the summary statistics. All correlations are adjusted for age. REMs rapid eye movements, std standard deviation, CV coefficient of variation, MoCA Montreal Cognitive Assessment, TMT Trial Making Test, NN normal-to-normal heartbeat interval, SDNN standard deviation of NN, HF high-frequency power normalized units (HFnu) were calculated as HF/(HF + LF)*100, LF/HF low frequency to high-frequency ratio, str stride, TUG Timed Up and Go test, reg regularity, DT dual-task (performing a cognitive task of serial subtraction of 3), dur duration.
REM density was negatively associated with stride time and TUG turn duration. Additionally, REMs intervals and REMs std were positively associated with TUG turn duration. These associations were observed exclusively under dual-task conditions (Fig. 2), indicating motor-cognitive interactions. Furthermore, lower REM density was also associated with poorer cognitive performance, as measured by TMT-B.
Eligible HRV data were obtained for 52 patients (Table 2). Among the HRV variables, SDNN was negatively associated with age and time to perform TMT-B, while a positive correlation was observed with disease duration (Fig. 2). None of the HRV measures were significantly associated with REM density or REMs interval variables. HRV variables were unrelated to the apnea-hypopnea index (AHI), periodic limb movement in sleep (PLMS) index, or arousal index (p > 0.102).
To further explore these associations in a subgroup of participants with complete data across all key measures, we provide Supplementary Fig. 1, which presents a correlation matrix based on the 45 participants with full datasets. While similar trends were observed, most correlations were no longer statistically significant, likely due to limited power. Scatter plots illustrating the relationships between REM density and dual-task TUG turn time and LF/HF ratio are provided in Supplementary Figs. 2, 3, respectively.
Given the potential influence of dopaminergic medication on HRV measures, we further evaluated the correlations between HRV and REMs measures separately for each medication group. No significant correlations were found either in the drug-naïve group (n = 10) or the daytime medication group (n = 33). However, in the evening slow-release medication group (n = 9), REM density was positively associated with HF (ρ = 0.91, p = 0.002) and negatively with LF/HF ratio (ρ = −0.91, p = 0.002). No other correlations were significant.
The effect of dopaminergic medication on REM density
Table 2 presents the comparison between the medication groups. The evening slow-release group included ten patients on levodopa CR, two on both levodopa CR and amantadine, one on the rotigotine patch, and three exclusively on amantadine. Patients on evening slow-release medication showed significantly higher REM density than those in the other two groups (p < 0.05, Fig. 3). As expected, drug-naïve patients had shorter disease duration and lower MDS-UPDRS III and bradykinesia scores than other groups. No significant differences were observed in any measures between the groups taking medications, except for a higher overall LEDD dose and higher ESS scores in the evening slow-release group (Table 2). Among participants receiving dopaminergic medication (n = 60), the total LEDD was not significantly correlated with REM density (Spearman ρ = 0.06, p = 0.64).
The evening slow-release medication group (n = 16) comprised patients taking evening controlled-release levodopa (CR, n = 10, black dots), evening CR levodopa combined with evening amantadine (n = 2, yellow dots), rotigotine patch (n = 1, blue dot), and evening amantadine alone (n = 3, green dots). *The daytime medication group included patients on diurnal or immediate-release evening medication. The evening slow-release medication group showed higher REM density compared to both the drug-naïve and daytime medication groups.
Discussion
To our knowledge, this study is the first to explore the relationship between REM density and motor, cognitive, and autonomic function in patients with PD. Our analysis highlights several points: (1) Lower REM density was associated with worse gait and mobility metrics mainly in the rhythm domain, especially under challenging conditions, highlighting the role of motor-cognitive interactions, (2) administration of evening slow-release dopaminergic medication, alleviating the motor impairments, resulted in higher (better) REM density, (3) autonomic function as assessed using HRV measures, was not associated with REM density in this PD cohort.
In this study, the MDS-UPDRS III bradykinesia score was negatively associated with REM density, linking impaired motor function to reduced REM density. However, contrary to our hypothesis, no significant associations were found between REM density and gait metrics directly reflecting the pace domain (i.e., gait speed). Contrarily, REM density was associated with impaired gait rhythmicity known to be impacted in early clinical phases20,21,27. Notably, the gait evaluation was performed in the ON-medication state. While dopaminergic treatment is known to improve certain gait domains, such as pace, its effects on rhythmicity and variability are limited27,28. This differential efficacy across gait domains could explain the variance in the relationship between the metrics.
In our study, REM density was not associated with traditional measures of sleep continuity (e.g., sleep efficiency, WASO, arousal index) or with subjective sleepiness as measured by the ESS. This suggests that REM density may reflect more specific neurophysiological mechanisms, rather than general sleep fragmentation or hypersomnolence. We propose that both reduced REM density and impaired complex gait performance may stem from shared dysfunction within brainstem and subcortical circuits, particularly those involving dopaminergic and cholinergic systems, which are implicated in both REM sleep regulation and motor-cognitive automaticity in PD.
Gait is a bilateral, coupled motion involving multiple joints and synchronized limb movements29. It follows a cyclic pattern, with alternating left and right leg movements ensuring smooth and rhythmic locomotion. The alternating REMs between leftward and rightward directions also resemble rhythmic cyclic patterns characteristic of gait (Fig. 1A–C). To explore this, we applied a novel approach to assess REMs rhythmicity and variability by calculating REMs interval and REMs CV. We found that the REMs interval and REMs std were positively associated with TUG turn duration DT. Turning is a complex task that requires force, balance, coordination and planning and processing of internal and external stimuli30. Patients with PD commonly take several short, rigid steps with reduced magnitude to turn instead of the fluid turning motion that is typical in healthy individuals reflecting reduced rhythmicity and increased variability21. These findings suggest that REM variability metrics have a similar pattern as complex, non-automatic movement.
A potential shared mechanism may involve dysfunction within basal ganglia-brainstem circuits, which are critically involved in the generation of both automatic motor patterns (e.g., gait) and phasic REM events. Specifically, degeneration of dopaminergic and cholinergic pathways, particularly involving the pedunculopontine nucleus and related structures, could disrupt stability and timing of rhythmic outputs in both motor and ocular systems31. As automaticity diminishes in PD, motor behaviors such as turning become more variable and reliant on top-down cognitive control; similarly, variability in REMs during sleep may reflect reliance on impaired automatic generation of phasic motor activity. Future studies using neuroimaging or neurophysiological measures of brainstem integrity could provide a test whether REMs variability can serve as a surrogate marker for the loss of motor automaticity in PD.
Gait and REMs also differ mechanistically. The bilateral coupled (inverse) motion of gait is enabled through the interconnected nature of joint movements in a closed kinematic chain, where the distal aspect of the extremity is fixed to an object that is stationary. This allows for effective force transmission, proprioception, motion feedback, and stability, all critical for maintaining rhythmicity and balance. In contrast, REMs, are synchronous movement of both eyes in the same direction (conjugate). REMs occur during REM sleep but can also occur during wakefulness when individuals scan their environment1. During wake, the environment provides feedback on movement and can help fix the gaze. Seemingly, there is no such constraint or feedback from the environment during sleep, although, recent work suggested that REMs reflect gaze shifts during dreams, associated with the cognitive processes of the sleeping brain32,33 which may suggest some fixation. Yet this theoretical suggestion requires further inquiry. Thus, the movement of the eyes is more varied and flexible than that reflected in an in-clinic gait assessment, which may explain the greater association with rhythmicity metrics than other gait metrics.
The connection between gait and eye movement is intriguing as these systems are often reliant on one another34. Interestingly, nystagmus, a condition in which the eyes make repetitive, uncontrolled movements with high REM density, is associated with impaired balance and coordination35. Pathophysiologically, central positional nystagmus is caused by cerebellar and/or brainstem dysfunction which is also affected by the neurodegenerative process in PD. The negative associations found in our study between lower REM density and measures from complex walking (i.e., turning) are particularly relevant, as turning is known to reflect impaired balance control and high fall risk21. The negative associations found in our study between lower REM density and complex gait tasks (i.e., turning) are consistent with prior reports linking reduced REM density to hyporarousal states like PD. Conversely, hyperarousal conditions such as depression have been associated with increased REM density. These observations motivate the speculative hypothesis of a non-linear, U-shaped relationship between REM density and arousal regulation, or more broadly, brain functional integrity. In this framework, moderate REM density may reflect a balanced arousal system that supports flexible cognitive and motor control, whereas both low and high levels could reflect maladaptive states. Future research is needed to formally test this non-linear relationship between REM physiology and motor control in PD.
While the rhythmic nature of REMs and gait invites comparison, we acknowledge that the neural control of REMs during sleep differs from that of voluntary saccades during wakefulness. Nevertheless, we suggest that both types of eye movements may share common underlying mechanisms, particularly within subcortical and brainstem circuits involved in automatic motor control. Notably, saccadic performance during wakefulness, especially increased latency and variability, has been linked to reduced motor automaticity and freezing of gait in PD, suggesting shared deficits in automaticity across ocular and locomotor systems36. Future studies combining eye-tracking during wakefulness with REM density analysis during sleep could help clarify whether reduced REM sleep eye movement metrics reflect broader impairments in oculomotor function.
Complex situations, such as dual-tasking or turning, are not purely motor functions but rather require both motor and cognitive resources21,22. Basal ganglia dysfunction in PD reduces automatic control of movements, increasing reliance on cognitive resources for walking, as demonstrated by greater prefrontal cortical activity during dual-task conditions37,38. As PD progresses, both gait and cognitive functions decline, making complex tasks increasingly challenging21. A significant association between lower REM density and poorer cognitive performance on the TMT-B, a test sensitive to executive function, further suggests cognitive involvement. This finding is consistent with prior research demonstrating an association between REM density and cognitive performance12,13. Gait and cognitive control share overlapping neural networks, particularly within the frontal subcortical circuits involved in executive function39,40. Furthermore, degeneration of basal ganglia circuits that project to the dorsolateral prefrontal cortex may contribute to the executive function deficits observed in PD41. Consequently, the association between reduced REM density and motor-cognitive interaction in PD may, therefore, reflect the impact of basal ganglia degeneration on REM density.
Degeneration of the cholinergic system, including brainstem structures such as the PPN, may underlie both the reduced REM density and the impaired motor-cognitive interaction observed in PD. The cholinergic system plays a central role in regulating REM sleep, arousal, and executive function. The PPN contributes to REM sleep generation, postural control, and gait initiation, and its degeneration has been linked to disturbances in REM sleep architecture and cognitive-motor performance42,43,44. Thus, the combined effects of cholinergic and dopaminergic dysfunctions in PD could therefore provide a common pathophysiological basis for the associations found in our study between reduced REM density, executive dysfunction, and deficits in gait under complex conditions.
Contrary to our expectations, no associations were found between HRV measures and REMs variables across the entire PD cohort. PD patients often present with lower HRV24, indicating an autonomic dysfunction. Furthermore, LF power spectrum values have been shown to be associated with MDS-UPDRS total and motor scores45. Nevertheless, there was no association between HRV measures and MDS-UPDRS III or bradykinesia scores in this study. These findings can possibly stem from the heterogeneous medication profile of our patients. Levodopa, a precursor of dopamine and norepinephrine, is likely to affect cardiovascular autonomic function46. Dopaminergic medications can influence HRV, with the effect varying based on the timing and dosage of the medication46,47. Interestingly, we found a strong association between REM density and HRV frequency domain in the evening slow-release medication group. This finding is based on a small sample size. Research on the specific impact of dopaminergic medication on nocturnal HRV is still insufficient.
Previous work has shown that lower REM density was associated with reduced HF, reflecting diminished parasympathetic activity, and a higher LF/HF ratio, indicating sympathetic dominance24. Typically, the non-REM sleep stage is characterized by a higher parasympathetic tone, whereas REM sleep involves a shift in sympathovagal balance from parasympathetic predominance toward increased sympathetic activity48. Our results support that evening dopaminergic medications may modulate the sympathovagal balance during REM sleep, aligning it with expected parasympathetic hypoactivity. Reduced REM density may indicate neurodegeneration in regions affected by PD, such as the brainstem (e.g., locus coeruleus, pedunculopontine nucleus) and hypothalamus, which are critical for REM sleep regulation and autonomic control49,50,51. Degeneration in these areas could impair parasympathetic modulation during REM sleep and disrupt the normal sympathovagal balance. Furthermore, the exclusive presence of these associations in the evening slow-release medication group may suggest an impact of dopaminergic treatment on autonomic function, warranting further investigation in larger cohorts. These alterations likely contribute not only to sleep disruption but also to the broader dysautonomia profile frequently observed in PD. The locus coeruleus (LC), a key noradrenergic nucleus, plays a critical role in autonomic regulation through its widespread noradrenergic projections, with distinct neuronal populations potentially exhibiting differential vulnerability to PD pathology based on their connectivity to motor or autonomic circuits52,53. While LC projections to the basal ganglia contribute to movement-related dysfunction, its connections to the hypothalamus and brainstem autonomic centers regulate cardiovascular and respiratory control. This neuronal heterogeneity may explain why some patients develop pronounced motor symptoms with minimal autonomic dysfunction while others experience early autonomic disturbances and why our initial hypothesis regarding autonomic correlations was only partially supported by the data.
Reduced REM density may already be evident in the early stages of PD, even before the initiation of dopaminergic treatment. This is supported by the comparable REM density values observed in drug-naïve patients and those not receiving evening slow-release dopaminergic medication, highlighting the impact of the neurodegenerative process on REM density reduction. Neurodegeneration may affect nocturnal motor control, often reflected by nocturnal hypokinesia. The administration of evening slow-release dopaminergic medications is prescribed to improve nocturnal motor control25. Interestingly, REM density was higher in patients receiving slow-release dopaminergic medication, suggesting that the medications effect on motor control also improved REM density. In contrast, the effects of immediate-release medications may not last into the second half of the night, when REM sleep and REM density are more prominent9,16. Furthermore, total LEDD was not correlated with REM density, aligning with our hypothesis that only evening-administered slow-release medications may influence REM expression, likely due to their pharmacokinetics and temporal proximity to REM-rich sleep periods.
The effect of dopaminergic medication on sleep is complex and depends on type, timing, and dosage. Low doses may act on presynaptic D2 receptors to promote sleep and reduce wakefulness26,54,55, whereas higher doses may activate postsynaptic D1/D2 receptors, potentially disrupting sleep and increasing insomnia55,56,57. Nonetheless, dopaminergic therapies can alleviate sleep disturbances in PD, including insomnia, fragmentation, and motor-related disorders58. Recent evidence links evening administration to improved sleep efficiency reduced WASO, and longer total sleep time26. Given this variability, future studies should aim to identify patient-specific predictors of treatment response, such as disease stage, receptor sensitivity, or genetic factors, to guide personalized sleep interventions in PD.
There is a shortage of evidence on the effect of PD medication on REM density. Pramipexole treatment for RBD symptoms in iRBD patients caused a reduced REM density in one study59, with no difference found in another study60. In another small study, the administration of a rotigotine patch to 11 advanced PD patients with RBD did not affect REM density61. Notably, most patients had already been receiving levodopa before the study. This could suggest that patients had reached a ceiling effect of the treatment before the introduction of rotigotine. Future research should establish the effects of multiple dopaminergic medications on REM density and explore how improvements in REM density influence motor and cognitive function.
In the present study, both groups taking medications underwent clinical and gait evaluation during a diurnal ON-medication state and had similar disease duration and severity. As a result, no significant differences in gait metrics were found between the two groups. Despite significant differences in REM density across the three groups, no differences were found in REMs interval and variability. This suggests that the temporal features of REMs might involve different underlying mechanisms compared to the overall REM density. Interestingly, REMs interval variables were unrelated to PD motor severity and may be more closely associated with cognitive impairment, as demonstrated by stronger correlations with TMT-B performance than REM density. Additionally, whether these REM features are altered in PD compared to healthy controls warrants further investigation.
An increase in REM density is often seen as an indicator of a reduction in sleep pressure or the satisfaction of a sleep need9. Consistent with this perspective, reduced REM density in PD compared to HC was proposed as a biomarker of excessive daytime sleepiness17, which is prevalent in PD19. Nevertheless, this study did not find associations between reduced REM density and excessive daytime sleepiness measured by the ESS scale, and REM density was independent of PSG sleep measures. This discrepancy could be partially explained by the side effects of medication on excessive daytime sleepiness. Although evening PD medication may increase REM density, some medications, especially dopamine agonists, could also contribute to increased daytime sleepiness62. Additionally, although the ESS is widely used in clinical practice, it relies on self-reports and exhibits weak associations with other behavioral and physiological sleep measures63.
The relationship between REM density and RBD remains unclear, with conflicting findings in the literature. For instance, PD patients with RBD exhibited higher REM density than those without RBD64, consistent with higher REM density in iRSWA participants than HC65. However, this contrasts with evidence of reduced REM density in PD compared to controls16,17. This study found no significant differences in REM density between PD patients with RBD and those without RBD, consistent with previous reports comparing iRBD and HC66,67. Furthermore, REM density was independent of RSWA%, a PSG marker of RBD severity. Including the RBDQ in the analysis did not alter the pattern of associations with REM density, providing additional support for the robustness of our findings.
For the first time, an association was shown between gait motor function and eye movement motor function during REM sleep. Our study included patients with and without RBD, enhancing its generalizability. Additionally, we showed that REM density was not associated with AHI and PLMS, both of which are prevalent in PD68. Nonetheless, our study has several limitations, namely the observational design of this study and the relatively small sample size. Furthermore, eight patients in this study were using SSRIs. While SSRIs are known to affect REM sleep, their impact on REM density remains poorly understood. Importantly, no significant differences in REM density were observed between patients taking SSRIs and those who were not. It is important to note that the evening slow-release medication group included a pharmacologically heterogeneous set of treatments, including amantadine. While amantadine may exert dopaminergic-like effects, its distinct mechanism of action and the small number of participants receiving it as monotherapy (n = 3) warrant cautious interpretation of this subgroup’s findings. More broadly, we acknowledge the limitations of subgroup analyses, particularly those involving small sample sizes, which should be interpreted with caution. Future studies should include drug-naïve patients to evaluate the effect of the administration of evening medication on REM density and explore the clinical significance of REM density within the context of PD progression. Additionally, evaluation of motor severity both during “on” and “off” medication states could provide a more comprehensive understanding of the underlying neurodegeneration. While several of our hypotheses were a priori and theory-driven, the broader set of correlation analyses was exploratory in nature and aimed at generating hypotheses for future studies. Thus, we performed multiple correlation analyses without applying formal corrections for multiple comparisons. As such, our findings should be interpreted with caution due to an increased risk of type I error. Furthermore, medications such as beta-blockers and calcium channel blockers, which are commonly used to treat hypertension and other non-cardiac conditions, can influence HRV by modulating autonomic tone. Although we excluded participants with overt cardiac disease from the HRV analysis, we did not systematically exclude or stratify based on the use of these medications. Future studies should include more detailed recording and subgroup analyses of autonomic-modulating medications, including antihypertensives, to better isolate their potential effects on HRV during REM sleep in PD.
These findings highlight the role of motor function, and motor cognitive interaction in the generation of rapid eye movements in PD. The administration of evening slow-release dopaminergic medications appears to increase REM density, likely by alleviating motor control deficits during REM sleep. As REM density is a potential biomarker of restorative sleep, we provide initial evidence that evening slow-release dopaminergic medication should be considered in PD. The relationship between REM density and autonomic function remains inconclusive, warranting further investigation.
Methods
Participants
Participants were recruited from the Laboratory for Early Markers of Neurodegeneration (LEMON) in the Neurological Institute at Tel Aviv Sourasky Medical Center. Patients with PD were included if they had a diagnosis of PD based on the MDS criteria69, had no history of other major neurological or psychiatric disorders (based on DSM IV), were not taking benzodiazepines, or antipsychotics, or had an implanted Deep Brain stimulation. Patients with PD and cardiac disease were included in the study but excluded from the HRV analysis. The study was approved by the Tel Aviv Sourasky Medical Center Ethics committee. Committee chair: Prof. Marcel Tupilski, MD; Committee officer: Ms Aneta David ( + 972 3 694 4678); application number: 202017839 approved on July 21st, 2020. All the participants provided informed written consent before participation.
Procedures
The study used an observational cross-section study design. On the day of the sleep assessment (polysomnography), all participants were instructed to avoid sleep medication, restrict the consumption of caffeine and alcohol, and adhere to their regular dopaminergic treatment schedule. All participants attended an additional visit within one day of the polysomnography (PSG). During this visit, demographic information was collected. The Montreal Cognitive Assessment (MoCA)70 was used to assess global cognition, and the Trail Making Test parts A and B (TMT–A, B) evaluated scanning and executive functions71. The Epworth Sleepiness Scale (ESS) was used to evaluate excessive daytime sleepiness (EDS)72, and the 10-item RBD Questionnaire (RBDQ) was used as a screening tool for RBD symptoms73. The MDS-UPDRS part III74 was administered by a certified movement disorders specialist while patients were in the ON-medication state, approximately 1 h after taking their medication. Bradykinesia, rigidity, and tremor subscales were calculated based on standard criteria, averaging the relevant items of MDS-UPDRS part III75. Information on PD medication types and intake times was collected and converted into Levodopa Equivalent Daily Dose (LEDD) using established criteria76.
Gait and mobility were evaluated using wearable sensors as previously described77. Briefly, participants wore five Inertial Measurement Units, including a tri-axial gyroscope, accelerometer, and magnetometer (OPAL system, APDM, Inc., Portland, OR, USA) on bilateral wrists, bilateral ankles, and lower back (L4-5 level). Participants were asked to walk back and forth in a ~ 15-meter corridor for 1 min under two conditions: (1) at their preferred, usual walking speed and (2) while performing a cognitive task of serial subtraction of 3 (dual task)77. Participants also performed the Timed Up and Go test (TUG), a performance-based measure of dynamic gait and balance78,79. The TUG was performed twice, once as a single task and once during a dual task (DT) (serial 3 subtraction).
Polysomnographic sleep recording (PSG)
Standard overnight PSG was conducted using a commercially available system (PSG Embla NDx, Natus). The montage consisted of a 6-channel EEG (F3, F4, C3, C4, O1, O2), two electrooculogram (EOG), and six electromyographic (EMG) electrodes affixed bilaterally on the mentalis, flexor digitorum superficialis, and tibialis anterior muscles80. A chest belt affixed with electrocardiography electrodes was used for ECG activity, and a nasal cannula registered respiratory status. All recordings lasted at least 6 h. Following the American Association of Sleep Medicine (AASM) guidelines, PSG was scored offline by a board-certified sleep medicine physician1. Standard sleep variables were automatically generated, including a detailed hypnogram and video assessment of REM to evaluate the existence of REM Sleep Behavior Disorder (RBD), known to be a marker of prodromal PD81. Previous work reported that PD patients with RBD show higher REM density than those without RBD64, potentially suggesting a different pathway in the neurodegenerative process. As such, we assessed REM sleep without atonia (RSWA) through visual detection from the EMG electrodes, with both tonic and phasic muscle activity events scored based on accepted guidelines82. The RSWA% was calculated as the percentage of 30-second REM sleep epochs meeting RSWA criteria out of all REM epochs. RBD was defined from video PSG assessment as previously described83,84. Apnea-Hypopnea Index (AHI), periodic limb movement in sleep (PLMS) index, and arousal index were also extracted.
Detection of rapid eye movements (REMs) during REM sleep
REMs were visually scored following established criteria1,11,85. Briefly, REMs were characterized by a rapid time course shorter than 500 ms, simultaneous appearance on both left and right EOG channels, and detectability above background noise, irrespective of amplitude. REM density was calculated as the number of REMs per minute of REM sleep.
REMs interval and REMs variability analysis
The manually detected eye movements were exported from the REM logic software and analysed using a custom-made MATLAB script. When a REM cycle was interrupted by more than 60 s of non-REM or wake, it was considered two separate cycles. REM cycles shorter than 3 min or containing fewer than five REMs were excluded from the analysis to avoid bias due to short or fragmented REM episodes. Similar to the approach used for HRV65, we calculated the average time intervals between consecutive REMs and the standard deviation (std) of these intervals. The time intervals between consecutive REMs were calculated separately for each REM sleep cycle. Additionally, we computed the coefficient of variation (CV) of the time intervals (std/mean*100), analogous to the method used in gait variability analysis86. The variables were averaged across all REM cycles in cases with multiple REM cycles.
Gait variables
Accelerometer and gyroscope signals from walking trials and the TUG tests were analysed using MATLAB proprietary algorithms77. For walking tasks, gait measures were calculated and categorized into three domains87: (1) Pace: gait speed, step length; (2) Rhythm: stride mean time; (3) Variability: stride time CV and stride regularity. For the TUG tasks, variables included TUG duration and turn duration.
Heart rate variability (HRV) analysis
For each participant, a single five-minute PSG segment recorded during REM sleep was selected and analysed for heart rate variability (HRV). Segments were carefully screened for apneas, arousals, complex movements, or ECG artifacts. HRV signal processing analyses were performed from the lead ECG signal (sampled at 250 Hertz) of the PSG, after confirming that the ECG channel was artifact-free. When applicable, the five-minute REM sleep segment was selected from the last REM period, as it is typically the longest and characterized by higher REM density16,23. This approach was chosen to standardize the HRV assessment duration and quality across participants and ensure artifact-free segments, allowing for consistent comparisons. The ECG data were exported from REM Logic into MATLAB and analysed using the HRVTool package88. Automatic RR interval detection was followed by manual inspection to ensure accuracy. Time domain measures, included in this study, were the normal-to-normal (NN) heartbeat interval and its standard deviation (SDNN). Frequency-domain analysis, which uses the Fourier transform to convert time-domain signals into frequency components, was also considered. Standard frequency domain measures were derived from the relative power spectral density of the low-frequency (LF) band (0.05–0.15 Hz), high-frequency (HF) band (0.15–4 Hz), and the LF/HF ratio24,65. For example, HF power normalized units (HFnu) were calculated as HF/(HF + LF)*100. These measures are proposed to represent different aspects of autonomic regulation: LF power reflects a combination of sympathetic and parasympathetic influences, HF power is associated with parasympathetic activity, and the LF/HF ratio is considered an indicator of sympathovagal balance24.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics version 29. Outliers were identified using the standard formula (Q3 + 3*IQR)89. The Shapiro-Wilk test assessed REM density normality, and nonparametric tests were applied when necessary. Data from participants presenting with less than 5 min of REM sleep during the PSG were excluded from the current analysis. Descriptive statistics are presented as mean ± std. Partial Spearman correlation analyses were performed to examine the relationships between gait, motor, cognitive, and HRV measures and REM density and REMs interval variables. Analysis was adjusted for age due to its impact on motor and cognitive functions. Given that dopaminergic medication may impact HRV measures, we conducted exploratory correlational analyses between HRV, REM density and REMs interval measures, stratifying participants into drug-naïve, daytime medication, and evening slow-release medication groups.
Since REM density is more prevalent in the second half of the night, we focused on evening medications likely to affect this period. The evening slow-release medication group included patients taking evening controlled-release levodopa (CR), the 24-h rotigotine transdermal patch90, or evening amantadine, which has a long half-life91. While amantadine is primarily an NMDA glutamate receptor antagonist, there is evidence that it also enhances dopaminergic transmission through both presynaptic (dopamine release) and postsynaptic (dopamine receptor sensitivity) mechanisms and may also inhibit dopamine reuptake91,92. REMs measures of this group were compared to drug-naïve patients and those taking daytime medication, defined as daytime medication or immediate-release evening medication26,56. Age and PD duration were included as covariates in each comparison using Nonparametric Quade ANCOVA. Additionally, demographic, clinical, gait, and HRV measures were compared between medication groups using the Kruskal-Wallis H test. In case of significance, post hoc analysis was applied using the Dunn test. Due to variable data availability (e.g., HRV, gait), each analysis included the maximum number of participants with valid data. Sample sizes are reported in the corresponding tables and figures.
Data availability
The data supporting the findings presented are available from the corresponding author upon reasonable request.
References
Berry, R. B. et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. www.aasmnet.org. (2015).
Ackermann, S. & Rasch, B. Differential effects of Non-REM and REM sleep on memory consolidation?. Curr. Neurol. Neurosci. Rep. 14, 430 (2014).
Tempesta, D., Socci, V., De Gennaro, L. & Ferrara, M. Sleep and emotional processing. Sleep. Med. Rev. 40, 183–195 (2018).
Howell, M. et al. Management of REM sleep behavior disorder: an American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep. Med. 19, 759–768 (2023).
Saleh, C., Diederich, N. J., Schroeder, L. A., Schmidt, M., Rüegg, S. & Khatami, R. Time to reconsider REM density in sleep research. Clin Neurophysiol 137, 63–65 (2022).
Ermis, U., Krakow, K. & Voss, U. Arousal thresholds during human tonic and phasic REM sleep: Phasic and tonic REM sleep. J. Sleep. Res. 19, 400–406 (2010).
Simor, P., van der Wijk, G., Nobili, L. & Peigneux, P. The microstructure of REM sleep: Why phasic and tonic?. Sleep Med. Rev. 52, 101305 (2020).
De Carli, F. et al. Activation of the Motor Cortex during Phasic Rapid Eye Movement Sleep. https://doi.org/10.1002/ana (2016).
Barbato, G. Is REM density a measure of arousal during sleep?. Brain Sciences 13, 378 (2023).
Arnaldi, D., Latimier, A., Leu-Semenescu, S., Vidailhet, M. & Arnulf, I. Loss of REM sleep features across nighttime in REM sleep behavior disorder. Sleep. Med. 17, 134–137 (2016).
Khalsa, S. B. S., Conroy, D. A., Duffy, J. F., Czeisler, C. A. & Dijk, D. J. Sleep- and circadian-dependent modulation of REM density. J. Sleep. Res. 11, 53–59 (2002).
Dykierek, P. et al. The value of REM sleep parameters in differentiating Alzheimer’s disease from old-age depression and normal aging. J. Psychiatr. Res. 32, 1–9 (1998).
Smith, C. T., Nixon, M. R. & Nader, R. S. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn. Mem. 11, 714–719 (2004).
Gilson, M. et al. REM-enriched naps are associated with memory consolidation for sad stories and enhance mood-related reactivity. Brain Sci. 6, 1 (2015).
Thase, M. E. et al. Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. Am. J. Psychiatry 154, 502–509 (1997).
Dagay, A. et al. Overnight distribution of REM sleep features in people with Parkinson’s Disease (PD) and Non-PD Controls. J. Parkinsons Dis. 13, 1213–1223 (2023).
Schroeder, L. A. et al. Reduced rapid eye movement density in Parkinson disease: a polysomnography-based case-control study. Sleep 39, 2133–2139 (2016).
Schapira, A. H., Chaudhuri, K. R. & Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450 (2017).
Dodet, P. et al. Sleep disorders in Parkinson’s disease, an early and multiple problem. NPJ Parkinsons Dis. 10, 46 (2024).
Bailo, G. et al. Characterization of walking in mild Parkinson’s disease: reliability, validity and discriminant ability of the six-minute walk test instrumented with a single inertial sensor. Sensors 24, 662 (2024).
Mirelman, A., Bonato, P., Camicioli, R., Ellis, T. D., Giladi, N., Hamilton, J. L. & Almeida, Q. J. Gait impairments in Parkinson's disease. Lancet Neurol. 18, 697–708 (2019).
Amboni, M., Barone, P. & Hausdorff, J. M. Cognitive contributions to gait and falls: evidence and implications. Mov. Disord. 28, 1520–1533 (2013).
Sorensen, G. L., Mehlsen, J. & Jennum, P. Reduced sympathetic activity in idiopathic rapid-eye-movement sleep behavior disorder and Parkinson’s disease. Auton. Neurosci. 179, 138–141 (2013).
Memon, A. A., George, E. B., Nazir, T., Sunkara, Y., Catiul, C. & Amara, A. W. Heart rate variability during sleep in synucleinopathies: a review. Front. Neurol. 14, 1323454 (2024).
Bhidayasiri, R., Sringean, J. & Thanawattano, C. Sensor-based evaluation and treatment of nocturnal hypokinesia in Parkinson’s disease: an evidence-based review. Parkinsonism Relat. Disord. 22, S127–S133 (2016).
Costa, E. C. et al. Sleep “ON”, sleep better! Positive effects of levodopa on sleep behaviour in people with Parkinson’s disease. J Sleep Res. https://doi.org/10.1111/jsr.14240 (2024).
Galna, B., Lord, S., Burn, D. J. & Rochester, L. Progression of gait dysfunction in incident Parkinson’s disease: Impact of medication and phenotype. Mov. Disord. 30, 359–367 (2015).
Lord, S., Baker, K., Nieuwboer, A., Burn, D. & Rochester, L. Gait variability in Parkinson’s disease: an indicator of non-dopaminergic contributors to gait dysfunction?. J. Neurol. 258, 566–572 (2011).
Park, K., Roemmich, R. T., Elrod, J. M., Hass, C. J. & Hsiao-Wecksler, E. T. Effects of aging and Parkinson’s disease on joint coupling, symmetry, complexity and variability of lower limb movements during gait. Clin. Biomech. 33, 92–97 (2016).
Miri, A. L. et al. A biomechanical analysis of turning during gait in individuals with different subtypes of Parkinson’s disease. Clin. Biomech.112, 106166 (2024).
Bohnen, N. I. & Albin, R. L. The cholinergic system and Parkinson disease. Behavi. Brain Res. 221, 564–573 (2011).
van den Berg, N. H. et al. Eye movements during phasic versus tonic rapid eye movement sleep are biomarkers of dissociable electroencephalogram processes for the consolidation of novel problem-solving skills. Sleep 46, zsad151 (2023).
Senzai, Y. & Scanziani, M. A cognitive process occurring during sleep is revealed by rapid eye movements. Science 377, 999–1004 (2022).
Koren, Y. et al. Vision, cognition, and walking stability in young adults. Sci. Rep. 12, 513 (2022).
Cao, Z. et al. Risk factors related balance disorder for patients with dizziness/vertigo. BMC Neurol. 21, 186 (2021).
Nemanich, S. T. & Earhart, G. M. Freezing of gait is associated with increased saccade latency and variability in Parkinson’s disease. Clin. Neurophysiol. 127, 2394–2401 (2016).
Wu, T. & Hallett, M. Neural correlates of dual task performance in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 79, 760–766 (2008).
Takakusaki, K., Oohinata-Sugimoto, J., Saitoh, K. & Habaguchi, T. Role of basal ganglia–brainstem systems in the control of postural muscle tone and locomotion. 231–237. https://doi.org/10.1016/S0079-6123(03)43023-9 (2004).
Rosso, A. L. et al. Aging, the central nervous system, and mobility. J. Gerontol. Ser. A 68, 1379–1386 (2013).
Parihar, R., Mahoney, J. R. & Verghese, J. Relationship of gait and cognition in the elderly. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2, 167–173 (2013).
Kelly, V. E., Eusterbrock, A. J. & Shumway-Cook, A. A review of dual‐task walking deficits in people with Parkinson′ s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinson’s Disease 2012, 918719 (2012).
Müller, M. L. T. M. et al. Clinical markers for identifying cholinergic deficits in Parkinson’s disease. Mov. Disord. 30, 269–273 (2015).
Boucetta, S., Cissé, Y., Mainville, L., Morales, M. & Jones, B. E. Discharge profiles across the sleep–waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 34, 4708–4727 (2014).
Fasano, A., Canning, C. G., Hausdorff, J. M., Lord, S. & Rochester, L. Falls in Parkinson's disease: a complex and evolving picture. Mov. Disord. 32, 1524–1536 (2017).
Haapaniemi, T. H. Ambulatory ECG and analysis of heart rate variability in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 70, 305–310 (2001).
Li, K. et al. Subthalamic nucleus stimulation and levodopa modulate cardiovascular autonomic function in Parkinson’s disease. Sci. Rep. 7, 7012 (2017).
Ruonala, V., Tarvainen, M. P., Karjalainen, P. A., Pekkonen, E. & Rissanen, S. M. (2015, August). Autonomic nervous system response to L-dopa in patients with advanced Parkinson's disease. In 2015 37th Annual International Conference of the IEEE Engineering in Medicineand Biology Society (EMBC), 6162-6165 (IEEE, 2015).
Chouchou, F. & Desseilles, M. Heart rate variability: a tool to explore the sleeping brain?. Front. Neurosci. 8, 402 (2014).
Kanda, T., Tsujino, N., Kuramoto, E., Koyama, Y., Susaki, E. A., Chikahisa, S. & Funato, H. Sleep as a biological problem: an overview of frontiers in sleep research. J. Physiol. Sci. 66, 1–13 (2016).
Paredes-Rodriguez, E., Vegas-Suarez, S., Morera-Herreras, T., De Deurwaerdere, P. & Miguelez, C. The noradrenergic system in Parkinson’s disease. Front. Pharm. 11, 435 (2020).
Spinassi, R. The Sentinel Sleep Theory: The Biological Function of REM Sleep Unveiled. https://doi.org/10.20944/preprints202408.1867.v1 (2024).
Gesi, M. et al. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci. Biobehav. Rev. 24, 655–668 (2000).
Bari, B., Chokshi, V. & Schmidt, K. Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease. Neural Regen. Res. 15, 1006 (2020).
Monti, J., Hawkins, M., Jantos, H., D’Angelo, L. & Fernandez, M. Biphasic effects of dopamine D-2 receptor agonists on sleep and wakefulness in the rat. Psychopharmacology 95, 395–400 (1988).
Zhu, K., van Hilten, J. J. & Marinus, J. The course of insomnia in Parkinson’s disease. Parkinsonism Relat. Disord. 33, 51–57 (2016).
Chahine, L. M. et al. Association between dopaminergic medications and nocturnal sleep in early-stage Parkinson’s disease. Parkinsonism Relat. Disord. 19, 859–863 (2013).
Tholfsen, L. K., Larsen, J. P., Schulz, J., Tysnes, O. B. & Gjerstad, M. D. Changes in insomnia subtypes in early Parkinson disease. Neurology 88, 352–358 (2017).
Liguori, C. et al. Rotigotine effect on sleep in a de novo Parkinson’s disease patient affected by periodic limb movement disorder. Parkinsonism Relat. Disord. 21, 1476–1478 (2015).
Sasai, T., Inoue, Y. & Matsuura, M. Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. Tohoku J. Exp. Med. 226, 178–181 (2012).
Fantini, M., Gagnon, J., Filipini, D. & Montplaisir, J. Brief Commun. www.submit.neurology.org (2003).
Wang, Y. et al. Effects of rotigotine on REM sleep behavior disorder in Parkinson disease. J. Clin. Sleep. Med. 12, 1403–1409 (2016).
Gandhi, K. D., Mansukhani, M. P., Silber, M. H. & Kolla, B. P. Excessive daytime sleepiness: a clinical review. In Mayo Clinic Proceedings, Vol. 96, 1288-1301 (Elsevier, 2021) https://doi.org/10.1016/j.mayocp.2020.08.033.
Lok, R. & Zeitzer, J. M. Physiological correlates of the Epworth Sleepiness Scale reveal different dimensions of daytime sleepiness. SLEEP Adv. 2, zpab008 (2021).
Zhu, J. et al. Increased rapid eye movement density in Chinese patients with Parkinson’s disease and RBD. Neurol. Sci. 42, 961–968 (2021).
Dijkstra, F. et al. Polysomnographic phenotype of isolated REM sleep without atonia. Clin. Neurophysiol. 131, 2508–2515 (2020).
Dauvilliers, Y. et al. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep 30, 844–849 (2007).
Montplaisir, J. et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov. Disord. 25, 2044–2051 (2010).
Thangaleela, S., Sivamaruthi, B. S., Kesika, P., Mariappan, S., Rashmi, S., Choeisoongnern, T. & Chaiyasut, C. Neurological insights into sleep disorders in Parkinson’s disease. Brain Sci. 13, 1202 (2023).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Sanchez-Cubillo, I. et al. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 15, 438–450 (2009).
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545 (1991).
Stiasny-Kolster, K. et al. The REM sleep behavior disorder screening questionnaire - A new diagnostic instrument. Mov. Disord. 22, 2386–2393 (2007).
Goetz, C. G. et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Horisawa, S. et al. Unilateral pallidothalamic tractotomy for akinetic-rigid Parkinson’s disease: a prospective open-label study. J. Neurosurg. 135, 799–805 (2021).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Mirelman, A. et al. Detecting sensitive mobility features for Parkinson’s disease stages via machine learning. Mov. Disord. 36, 2144–2155 (2021).
Podsiadlo, D. & Richardson, S. The Timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148 (1991).
Shumway-Cook, A., Brauer, S. & Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys. Ther. 80, 896–903 (2000).
Iranzo, A. et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep. Med. 12, 284–288 (2011).
Zhang, H. et al. Risk factors for phenoconversion in rapid eye movement sleep behavior disorder. Ann. Neurol. 91, 404–416 (2022).
Cesari, M. et al. Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: guidelines from the International RBD Study Group. Sleep 45, zsab257 (2022).
Dauvilliers, Y., Schenck, C. H., Postuma, R. B., Iranzo, A., Luppi, P. H., Plazzi, G. & Boeve, B. REM sleep behaviour disorder. Nature reviews Disease primers 4, 19 (2018).
Sateia, M. J. International Classification of Sleep Disorders-Third Edition. Chest 146, 1387–1394 (2014).
Leclair-Visonneau, L., Oudiette, D., Gaymard, B., Leu-Semenescu, S. & Arnulf, I. Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain 133, 1737–1746 (2010).
Hillel, I. et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur. Rev. Aging Phys. Act. 16, 6 (2019).
Lord, S., Galna, B., Verghese, J., Coleman, S., Burn, D. & Rochester, L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J. Gerontol. Series A Biomed. Sci. Med. Sci. 68, 820–827 (2013).
Vollmer, M. HRVTool - an Open-Source Matlab Toolbox for Analyzing Heart Rate Variability. in 2019 Computing in Cardiology Conference (CinC) 45 (Computing in Cardiology, 2019).
Hoaglin, D. C. John W. Tukey and data analysis. Stat. Sci. 18, 311–318 (2003).
Frampton, J. E. Rotigotine transdermal patch: a review in Parkinson’s Disease. CNS Drugs 33, 707–718 (2019).
Rascol, O., Fabbri, M. & Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. The Lancet Neurol. 20, 1048–1056 (2021).
Connolly, B. S. & Lang, A. E. Pharmacological Treatment of Parkinson Disease. JAMA 311, 1670 (2014).
Acknowledgements
The authors would like to thank the participants of the study for their time and effort. This project was supported by the USA MED Research Acquisition Activity, Department of Defense awarding office, through the FY19 Award Mechanism: Parkinson’s Research Program Investigator-Initiated under Award No. W81XWH2010468. The funding agency was not involved in the design, acquisition, interpretation, or the findings or the writing of this manuscript. AD is a Ph.D. student supported in part by the Aufzien Family Center for the Prevention and Treatment of Parkinson's Disease Grant and the Israeli Scholarship Education Foundation (ISEF) for excellence in academic and social leadership.
Author information
Authors and Affiliations
Contributions
A.D.: Execution, statistical analysis, writing the manuscript; S.K.: Data collection, critique of manuscript; N.E.: Data collection, critique of manuscript; J.V.: Data processing, critique of manuscript; N.G.: Conception, interpretation of initial findings, review of the manuscript; R.T.: Review, critique of manuscript; J.H.: Review, data interpretation, critique of manuscript; A.M.: Conception, obtained funding, data interpretation review, writing the manuscript; J.Z.: Data interpretation review, writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
AD, SK, NE, JV, RT, NG, and JZ report no COI pertaining to the current project. AM reports receiving a grant from the USA MED Research Acquisition Activity, Department of Defense (DOD) to support this research as well as grants from MJFF and JPND. She does not have any COI pertaining to this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dagay, A., Katzav, S., Elisha, N. et al. REM density in Parkinson’s disease: association with motor, cognitive, autonomic function, and dopaminergic medication. npj Parkinsons Dis. 11, 211 (2025). https://doi.org/10.1038/s41531-025-01057-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01057-1