Abstract
This research aimed to establish a model predicting mild cognitive impairment in Parkinson’s disease patients (PDMCI) by integrating multimodal indicators. We prospectively collected general demographic data, clinical scales, gait parameters, eye tracking parameters, and neuroimaging parameters from 50 PDMCI patients, 50 Parkinson’s disease patients with normal cognition (PDNCs), and 20 healthy controls (HCs). Support Vector Machine (SVM) classifiers and nested cross-validation were used to evaluate 31 feature combinations. Results demonstrated that the combination of clinical, gait, eye tracking, Diffusion Tensor Image Analysis along the Perivascular Space (DTI-ALPS), and Global Functional Connectivity Density (gFCD) features achieved an average accuracy of 0.9135 and an average area under the curve of 0.9602 on the test dataset. Notably, the combination of eye tracking and gait features also showed superior performance. These findings indicate that multimodal data integrated with machine learning (ML) can effectively distinguish between PDMCI and PDNC patients.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most frequent neurodegenerative disease. Its clinical presentations include motor symptoms, such as tremors, bradykinesia, and rigidity, as well as a broad spectrum of nonmotor symptoms, with cognitive impairment being a central issue that greatly affects the quality of life of patients1. Approximately 30%-80% of PD patients experience varying degrees of cognitive decline during the course of their disease, and ultimately, approximately 50% will develop Parkinson’s disease dementia (PDD)2. Nonetheless, the pathogenesis of mild cognitive impairment in PD (PDMCI) is complex, and its early detection and accurate evaluation remain challenging. Consequently, constructing a PDMCI prediction model through the integration of multimodal data holds substantial importance.
In PD patients, an elevated risk of cognitive impairment is strongly associated with several clinical and demographic characteristics, including an earlier age of onset3, lower educational attainment, the male sex4, higher Unified Parkinson’s Disease Rating Scale (UPDRS) scores (particularly for the verbal and postural stability components), and higher Hoehn–Yahr stages. Recent developments in eye-tracking technology and wearable gait monitoring devices have provided new insights into PD-associated cognitive impairment (PDCI). Research has demonstrated that PD patients exhibit reduced gait speeds5, shortened stride lengths, increased gait variability, and other features that are more pronounced in individuals with cognitive dysfunction6,7. During eye movement fixation tasks, PD patients typically display shorter maximum fixation durations and elevated saccade rates, suggesting that they have heightened levels of susceptibility to external distractions and difficulty maintaining stable fixation8. This fixation instability may be linked to cognitive dysfunction; however, the precise mechanisms underlying it remain to be elucidated. Neuroimaging is essential for both diagnosing PDMCI and predicting the prognosis of patients. According to our earlier study9, individuals with PDMCI show notable changes in global functional connectivity density (gFCD) in particular regions of the brain, encompassing the left calcarine fissure cortex and the right hippocampus. These changes may be associated with the pathophysiological mechanisms that underlie cognitive dysfunction. Furthermore, dysfunction of the lymphatic system in PD patients may exacerbate the pathological α-synuclein (α-Syn) accumulation, thereby worsening cognitive impairment10. Diffusion tensor imaging analysis along perivascular spaces (DTI-ALPS) indirectly evaluates glymphatic system functioning by assessing water molecules’ diffusion within these spaces in the brain11. Research has shown that PD patients have considerably lower DTI-ALPS scores, which is associated with both motor symptoms and cognitive dysfunction12,13.
In recent years, machine learning (ML), which can automatically learn and discover rules from extensive datasets, has been increasingly integrated into the medical domain. Specifically, ML has yielded significant advancements in disease diagnosis, prognosis prediction, and treatment plan optimization research14,15. With respect to the prediction of cognitive dysfunction, ML technology enables the precise identification of high-risk patients by leveraging multiple data sources16,17, thereby facilitating the use of early intervention strategies. In our prior research endeavours, we used ML methodologies to construct a model for predicting PDCI. For example, through the integration of proteomic and metabolomic analyses, we identified potential biomarkers associated with PDCI18. Another study conducted by our team9 used support vector machines (SVMs) to differentiate between PD patients with normal cognitive function (PDNC) and PDMCI patients by incorporating clinical data, blood markers, and neuroimaging information to achieve notable classification performance. Chen et al.19 employed machine learning algorithms such as decision trees, random forests, and XGBoost models, and based on the voxelin-voxelin-inter-voxelin-indicators extracted from diffusion tensor imaging (DTI), verified that these indicators could be effectively used to distinguish PDMCI from PDNC patients. Among them, the XGBoost model that combines in-voxel and inter-voxel metrics performed the best, with a test set accuracy of 91.67% and an AUC of 0.94. SHAP analysis indicated that the local diffusion consistency of the brainstem and the average diffusion rate of the right cingulate gyrus (hippocampus) were important features.Based on the data from the Early Longitudinal Study of Parkinson’s Disease in Singapore, Mohammadi et al.20 employed machine learning methods such as AutoScore, Random Forest, K-Nearest Neighbors and Neural Network. Its effectiveness in predicting the risk of cognitive decline within five years in patients with early-stage PD was verified. The study selected key predictors from baseline demographics, clinical evaluations, and blood biomarkers through variable selection and found that the Random Forest method performed the best (AUC value was 0.93, 95% CI: 0.89, 0.97), indicating that these machine learning models can effectively identify high-risk individuals with cognitive decline among early-stage PD patients, thereby supporting targeted intervention and improvement in PD management.
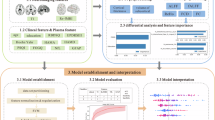
In this study, a model for predicting PDMCI based on multiple feature combinations was developed through the use of ML technology to integrate clinical, gait, eye tracking, and neuroimaging features. The performance of the classifier was evaluated, the model results were interpreted (Fig. 1), and the contributions of the multimodal indicators to the PDMCI prediction process were analysed. This study’s objective provided a new perspective on the discovery of putative indicators for PDMCI.
Results
Analysis of general demographic and clinical scale characteristics
Table 1 presents the results of the clinical data analyses for the three study groups. The patients in the PDMCI group were notably older than those in the HC and PDNC groups. Additionally, the patients in the PDMCI group presented lower levels of education as well as lower MoCA scores. Conversely, they produced higher scores on the UPDRS III, Hoehn-Yahr, HAMA, HAMD, NMSS, PDQ-39, ADL, and SCS-PD scales. The differences among these indicators were statistically significant. However, no statistically significant sex, disease duration, LEDD, PSQI score, or RBDSQ score differences were observed. We conducted a LASSO regression analysis of clinical scale scores and general demographic data (16 variables total) for the PDMCI and PDNC groups (Fig. 2a, b). The optimal lambda value (lambda.min) was selected, resulting in the identification of 14 key variables. These variables demonstrated significant predictive power in the model. The RF algorithm was subsequently employed to identify the features that had the greatest influence on the model; this revealed that education level had the most significant impact.
a, d, g, j illustrate the coefficient path plots for clinical data, gait, eye tracking and gFCD, respectively. b, e, h, k represent binomial deviations for clinical data, gait, eye tracking, and gFCD, respectively. c, f, i, l represent random forest algorithms for clinical data, gait, eye tracking, and gFCD, respectively.
Analysis of gait parameters in dual tasks
Table 2 summarizes the outcomes of a gait parameter analysis conducted under dual-task conditions. Compared with the PDNC group, the PDMCI group presented a reduced gait speed, decreased maximum angles of backswing of the left and right legs, and a lower average angular velocity while turning across different paradigms. Conversely, the average duration and number of steps during turning increased. In the narrow path model, relative to the HC group, the PDMCI group demonstrated decreases in the swing phase duration, maximum trunk sway angle, maximum backswing angles of both arms, arm swing amplitude, and average angular velocity during steering. There were also increases in the support phase duration, phase coordination index, and average steering time while walking. These differences were statistically significant. However, the arm symmetry index, the maximal forward swing angles of the left and right arms, and the left–right foot coordination did not vary significantly. For the LASSO regression analysis, a total of 33 variables were used. The performance achieved with various regularization parameters λ was evaluated using 100-fold cross-validation, with lambda.min selected as the optimal regularization parameter (Fig. 2e). In this way, five key variables were identified. The RF algorithm was subsequently employed to assess the feature importance levels of these variables (Fig. 2f); this revealed that the maximum posterior swing angles of the left and right legs in the narrow path paradigm had the most significant impact on the model.
Analysis of the characteristics of single-target and multitarget eye tracking indicators
Table 3 displays the outcomes of an analysis of the single-target and multitarget eye-tracking indicators. The PDMCI group had notably greater trial durations and reaction times, along with a significant decline in accuracy, as compared to the PDNC group. However, the total numbers of blinks per trial showed no obvious difference between the two groups.
To further analyse the data, we randomly selected one subject from each of the PDMCI, PDNC, and HC groups and observed the visualization results concerning fixation points, saccades, and blinks during both the single-target (20 trials) and multitarget (30 trials) eye tracking tasks. The findings revealed that the patients in the PDMCI and PDNC groups displayed more disordered saccade patterns than did those in the HC group, with most saccades in the HC group originating from the centre of the visual field (Fig. 3k). Additionally, the patients in the PDMCI group exhibited a significant blink frequency reduction relative to those contained in the PDNC group (Fig. 3i and l).
a Single-target eye tracking. b Multitarget eye tracking. c Accommodative pupil response and the CR threshold. d Nine-point calibration. e Nine-point verification. f Trial presentation. g Single-target eye-tracking fixation points (left: PDMCI, middle: PDNC, and right: HC; the rest of the pictures in this order). h Single-target eye-tracking saccades. i Single-target eye-tracking blinks. j Multitarget eye-tracking fixation points. k Multitarget eye-tracking saccades. l Multitarget eye-tracking blinks. m, n, and o Multitarget eye-tracking visualization heatmaps of the PDMCI, PDNC, and HC groups, respectively.
On the basis of the provided eye-tracking heatmap (Fig. 3m–o), we can discern distinct subject performance differences across different groups in the multitarget eye tracking task. The red areas in the heatmap indicate the regions with the highest attentional focus levels. The individuals in the PDMCI and PDNC groups clearly exhibited less stable attention than did those in the HC group.
Sixteen variables were initially included in the LASSO regression analysis; of these, eight key variables were selected (Fig. 2g, h). The RF algorithm was subsequently employed to assess the importance of these key variables (Fig. 2i). The evaluation results revealed that the accuracy of the multitarget eye tracking task had the most significant impact on the model.
Analysis of the characteristics of DTI-ALPS
An intergroup difference analysis of the left diffusion tensor imaging index along the perivascular space (L_ALPS_Index) revealed that the L_ALPS_Index was notably lower in the PDMCI group (median: 1.39) than in either the PDNC group (median: 1.49) or the HC group (median: 1.52) (p = 0.011).
Analysis of the characteristics of gFCD patients
In this research, we utilized an automated anatomical labelling (AAL) template to categorize brain regions. The AAL template encompasses 116 distinct brain regions; it includes 90 regions associated with the cerebral cortex and subcortical structures, as well as 26 regions pertaining to the cerebellum. Each brain region within the AAL template is assigned a unique identifier; for instance, identifier 46 corresponds to the right cuneus (Cuneus_R), which is situated in the occipital lobe and functions primarily in visual information processing cases. For clarity and conciseness, we refer to these brain regions by their respective identifiers throughout the text. A LASSO regression analysis was conducted on all 116 brain regions, resulting in the identification of 11 key regions (Fig. 2j, k). The RF algorithm was subsequently utilized to assess the feature importance levels of these key regions (Fig. 2l). The findings indicated that the right precuneus (identifier 68) had the most significant influence on the model.
Evaluation of the SVM classifier
During the preceding feature selection process, five key features were identified and used to construct the SVM classifier. To investigate the impacts of various feature combinations on the performance, a total of 31 classifiers were constructed and evaluated. Table 4 presents the performance evaluation results produced by the SVM classifier under different feature combinations. The performance of the classifier was assessed on the basis of several critical metrics, including average accuracy (ACC), the average area under the curve (AUC), average sensitivity, and average specificity. The results indicated that the feature combination that yielded the best performance for the classifier on the test set was “clinical + gait + eye tracking + DTI-ALPS + gFCD”; this combination achieved an average ACC of 0.9135, an average AUC of 0.9602, an average sensitivity of 0.9030, and an average specificity of 0.9240 (Fig. 4a, Model 1). Notably, the combination of “gait + eye tracking” features also demonstrated superior performance; it achieved an average ACC of 0.8480, an average AUC of 0.9053, an average sensitivity of 0.8500, and an average specificity of 0.8400 (Fig. 4b, Model 2). An analysis of the resulting confusion matrix revealed that while the model maintained high overall accuracy, it had certain limitations in terms of distinguishing between these two categories.
a, b Model 1 (clinical data + gait + eye tracking + DTI-ALPS + gFCD) and Model 2 (gait + eye tracking), respectively. The model’s average ROC curve is represented by the coloured dashed line, a random guess ROC curve is represented by the grey dashed line, and the model’s standard deviation range is shown by the shaded region. Among the 30 tested samples, Model 1 accurately classified 14 PDNC samples and 12 PDMCI samples, resulting in 2 false negatives (PDMCI misclassified as PDNC) and 2 false positives (PDNC misclassified as PDMCI). Model 2 correctly identified 12 PDNC samples and 12 PDMCI samples, with 3 false negatives and 3 false positives.
Fig. 5 presents a SHAP summary plot elucidating the prediction outcomes of the ML model. The general model with the best comprehensive performance incorporated clinical, gait, eye tracking, and quantifiable imaging indicators. Five key features are highlighted in the figure, ranked in descending order based on their significance to the predictive accuracy of the model, they are M.ACC, education level, N.SBSM, gFCD (68), and L_ALPS_Index.
Discussion
This study represents a pioneering effort to integrate clinical, gait, eye tracking, and neuroimaging indicators for the construction of a diagnostic model for PDMCI. Our findings indicate that gait abnormalities, eye-tracking disorders, a reduced L_ALPS_Index, and gFCD value alterations (indicating either heightened or diminished neural activity in particular areas of the brain) are connected with PDMCI. The most critical features derived from the five modules were selected and incorporated into ML algorithms in various combinations, and the model that exhibited the best overall performance was constructed. However, the model involving gait + eye tracking also had a mean AUROC of 0.9.
In a systematic analysis of features of clinical patients with PDMCI and PDNC, educational attainment emerged as the most significant demographic indicator for distinguishing between these two patient groups. Moreover, the PDMCI group presented significantly higher scores on the NMSS and PDQ-39, indicating a strong correlation between cognitive impairment and a greater severity of nonmotor symptoms, such as depression, anxiety, and apathy, as well as a diminished quality of life. Notably, the Hoehn–Yahr stage of the PDMCI group was much higher than that of the HCs, indicating a link between disease severity and cognitive deterioration. However, no obvious differences in the length of the disease or LEDD were observed between the two groups, implying that cognitive decline is not solely attributable to a prolonged disease duration or to the use of medication but may be linked to other underlying pathophysiological mechanisms of PD21.
In this study, gait assessments were conducted on three distinct subject groups utilizing wearable technology. The findings indicated that both the PDMCI and PDNC groups presented significantly lower kinematic parameters than did the HC group. These parameters included reduced gait velocities, diminished leg swing angles, decreased trunk sway angles, reduced arm swing amplitudes, and impaired steering and turning capabilities. These results suggest a prevalent impairment of motor function in patients with PD. Motor dysfunction is particularly pronounced in PD patients during complex tasks such as walking through a narrow corridor and turning. Compared with the HCs, the PDMCI and PDNC groups presented significant reductions in their gait speeds, average turning times, and average angular velocities when turning across various paradigms. Notably, in the dual-task narrow path paradigm, the PDMCI group performed worse than did either the PDNC or the HC group. This may be attributed to the increased complexity of the task and the heightened cognitive load associated with it; this reflects severe balance, visuospatial ability, and gait automation impairments in the PDMCI group. As a more complex and demanding task, the dual-task narrow path paradigm required patients to perform additional cognitive tasks while navigating through a confined space. This not only heightened the demand for sensory‒motor integration22 but also accentuated disease manifestation and symptomatology differences, thereby increasing the sensitivity of the assessment and diagnosis processes. In summary, gait parameters, as quantifiable and objective metrics, show promise as possible indicators for the early diagnosis of PDMCI, making it easier to identify people in the early stages of the illness and enabling timely interventions.
The eye-tracking parameter data collected in this investigation showed that the performances of the PD and HC groups were notably different in eye-tracking activities. These results imply that PDMCI is connected with impaired attention maintenance and allocation, as well as with compromised executive function. Such functional deficits are key indicators of cognitive dysfunction and may be closely linked to alterations in the functional connection of the thalamocortical network23, the salience network (SAL), and the frontoparietal network (FPN)24. Patients with PDNC also present issues such as attentional concentration disorders and reduced task execution efficiency. Although PDNC patients do not yet demonstrate significant cognitive impairment, abnormalities in their oculomotor parameters may serve as early indicators of PD. According to the eye-tracking heatmaps obtained in this study, the subjects in the HC group (Fig. 3o) maintained high attentional stability across all 30 trials, primarily focusing on the central area of the visual field. This suggests that they effectively utilized both their central and peripheral vision to continuously monitor the location of the target point. Compared with the HCs, the PDNC group (Fig. 3n) presented a slight attentional concentration reduction but maintained adequate focus, albeit with less stability than that in the HC group. The subjects in the PDMCI group (Fig. 3m) demonstrated significantly fragmented attention, as evidenced by the multiple red areas in the figure, indicating difficulty in sustaining prolonged visual fixation on the screen centre. In summary, the subjects in the HC group exhibited robust attentional stability during the multitarget eye tracking task, whereas those in the PDMCI group presented the poorest stability, with the PDNC group falling between these two extremes. These findings may reflect visual attention and oculomotor control differences among the groups and suggest that the PDMCI group may exhibit more pronounced visual attention deficits. One of the core features of PD is bradykinesia, which is characterized by reduced speeds and amplitudes in repetitive movements25. Research has indicated that PD patients exhibit lower spontaneous blink rates than do healthy controls26. Michaela et al.27 investigated both conscious rapid blinking and spontaneous blinking frequencies in PD patients and HCs and found notable differences between the two groups in the frequencies of both types of blinking, with rapid blinking demonstrating greater diagnostic accuracy for differentiating PD patients from HCs. In our study, the PDMCI group presented a significantly lower blink rate than did the PDNC group, as illustrated by both the single-target and multitarget eye tracking visualizations in Fig. 3i, l. However, the final comparison revealed that the total number of blinks did not differ between the two groups. Our trial’s design could be to blame for this disparity. Specifically, the durations of the individual trials (the time from stimulus presentation to button pressing) were not strictly controlled. Given that the PD patients typically exhibited longer reaction times during the trials, this extended duration may have partially compensated for the reduced blink rate, thereby obscuring any potential significant differences in the total numbers of blinks. In conclusion, eye tracking parameters hold promise as potential markers of cognitive decline in PD patients and could serve as valuable markers in future clinical trials conducted to evaluate disease-modifying therapies and cognitive enhancement interventions.
Consistent with the findings of previous studies28,29, our study demonstrated that the L_ALPS_Index was notably lower in patients with PDMCI than in those with PDNC or in HCs (p = 0.011). These findings imply that improving glymphatic function may be a viable treatment avenue for postponing the advancement of the disease and that the DTI-ALPS index may be used as a possible marker for PDMCI and the disease development. The participants in this study were exclusively right-handed individuals30, ensuring that their left cerebral hemispheres played a dominant role in their language and logical functions. The anatomical structures of the corticospinal tract and other fibre tracts are more clearly defined in right-handed individuals, and this enhances the accuracy of delineating the left ROI in DTI scans. Additionally, the periventricular vascular spaces in the left hemisphere exhibit greater contrast in DTI images, facilitating reduced measurement errors and improved data reproducibility. Consequently, the L_ALPS_Index was chosen as the evaluation metric for glial lymphatic system function. Through the gFCD analysis conducted in this study, it was observed (Fig. 6) that, compared with the PDNC group, the patients with PDMCI presented significantly greater gFCD values in the left thalamus, which is a finding that may be indicative of a cognitive compensation mechanism in these individuals. Reduced gFCD values in the left superior occipital gyrus and the right precuneus most likely indicate neurodegenerative alterations linked to cognitive decline. These gFCD alterations may serve as a potential neural basis for determining cognitive impairment in PDMCI patients, and they suggest promising targets and directions for future research and clinical interventions. However, given the limited number of studies in this area and their inconsistent conclusions, further investigation is warranted.
3, Left dorsolateral superior frontal gyrus; 12, Operculum of the left inferior frontal gyrus; 23, Left medial superior frontal gyrus; 25, Left orbital middle frontal gyrus; 33, Left medial and lateral cingulate gyri; 49, Left superior occipital gyrus; 68, Right precuneus; 70, Right paracentral lobule; 77, Left thalamus; 92, Crus I region of the right cerebellum; 100, Right cerebellar lobe 6.
On the basis of the findings described herein, we used SVMs to develop a multifeature ensemble ML model that can be used to differentiate between PDMCI and PDNC patients. Among the 31 classifiers evaluated, Classifier 1, which integrated features from “clinical data, gait analysis, eye movement metrics, DTI-ALPS, and gFCD”, demonstrated superior overall performance. The strength of this classifier lies in its multimodal data integration scheme, which enables a more comprehensive representation of PD characteristics and enhances the accuracy of PDMCI prediction. Notably, the models based solely on gait and eye movement features also exhibited strong independent predictive capabilities. Eye movement parameters can indicate decreases in visual attention and executive function levels, whereas gait indicators highlight reductions in motor and cognitive dual-task performance. Combining these features with others significantly improved the performance of the classifiers. Although Classifier 1 achieved the highest performance metrics, the combination of eye movement and gait features has greater practical value in clinical applications, particularly in primary care settings with limited resources. For example, eye tracking can be efficiently performed using portable devices, whereas gait analyses can be dynamically monitored through wearable sensors, thereby diminishing the dependence of the process on sophisticated imaging technologies. This streamlined and accessible feature acquisition approach offers a cost-effective and efficient early screening method, thus aiding in the expansion of early detection coverage for PDMCI. Moreover, compared with scales such as the MoCA, eye movement and gait metrics are more objective and quantifiable and thus effectively minimize subjective assessment biases31. In conclusion, the ease of accessibility and high resolutions of eye movement and gait features render them highly suitable for clinical diagnosis tasks, and these features thus provide a practical and reliable tool for PDMCI prediction.
The SHAP analysis indicates that eye movement characteristics (especially M.ACC) play a crucial role in the model, with their SHAP value distribution being wide, showing both positive and negative influences on the model’s predictions. Education level is negatively correlated with the model output. Low education level may increase the risk of PDMCI, suggesting that it is not only a cognitive indicator but also related to confounding factors such as socioeconomic status, occupational exposure, and lifestyle. Low education level may limit an individual’s ability to access health resources, affecting their disease cognition and management, and thereby indirectly exacerbating cognitive function decline. Gait characteristics (especially N.SBSM) have a significant positive contribution to the model prediction, reflecting an individual’s movement adaptation ability and balance control level in complex environments. Abnormal changes in gFCD in the right precentral gyrus have a significant positive impact on the model prediction, indicating that the alteration in the functional connectivity density of this brain region is a potential biomarker for PDMCI. The DTI-ALPS index has a negative regulatory effect on the model prediction, with lower values indicating a higher risk of PDMCI, which may be one of the pathological bases for cognitive decline. SHAP analysis provides strong guidance for clinical decision-making. It quantifies the contribution of each feature to the prediction model, helping to identify key risk indicators. Features with high SHAP values (such as M.ACC, N.SBSM, gFCD, and L_ALPS_Index) are closely related to the occurrence and development of PDMCI and can serve as potential markers for early diagnosis and intervention. Combined with the results of SHAP analysis and clinical accessibility, gait and eye movement features (M.ACC and N.SBSM) are easy to obtain and have significant contributions to the model, making them the preferred choice for early screening and facilitating their promotion in different medical scenarios. Moreover, SHAP analysis helps in feature dimensionality reduction, prioritizing the retention of high-contributing features, and constructing an efficient prediction model, enhancing the model’s interpretability and clinical practicability.
Although the multimodal model developed in this study demonstrated high diagnostic efficiency, several limitations warrant attention. First, the complexity associated with the multidimensional data collection process constrained the sample size to 50 cases each for PDMCI and PDNC setting. To confirm the generalizability of the model created, the sample size should be increased in future studies, and extend the observation duration. Second, this study is based solely on data collected from a single center and lacks external validation datasets. Future research should focus on promoting multi-center collaboration and validate the model through diverse independent datasets to enhance its robustness and applicability in clinical practice. Third, deep learning methods (such as neural networks) and ensemble learning methods (such as XGBoost, LightGBM, etc.) have demonstrated significant advantages in capturing complex patterns and dealing with high-dimensional feature spaces. These methods can fully utilize the complex features in the data, possess strong nonlinear modeling capabilities, and enhance generalization performance through optimization algorithms, thereby achieving precise predictions. However, they usually require a large amount of data for training to fully exploit their potential and reduce the risk of overfitting. Due to the limited sample size in this study, it may not be possible to fully unleash the potential of these methods, and thus, they were not included. Future research can expand the sample size to further explore and verify the efficacy of these methods, thereby optimizing the performance of the prediction model and better addressing the challenges of large-scale datasets. Fourth, the computation of neuroimaging indicators such as DTI-ALPS depends on the use of specific software and manual ROI mapping, and this introduces potential human biases. The development of automated image processing algorithms or the use of AI-assisted segmentation techniques could increase the objectivity and reproducibility of the data. Additionally, while this study incorporated data from multiple modalities, other potential biomarkers and clinical indicators, such as blood biomarkers9,18 and genetic markers32, could be integrated into the model to further improve its diagnostic accuracy.
In conclusion, this study developed an ML model that can effectively distinguish between PDMCI and PDNC patients by combining multimodal data. Notably, the combination of clinical + gait + eye tracking + DTI-ALPS + gFCD achieved an optimal balance between diagnostic accuracy and clinical feasibility, thereby offering a viable solution for the early detection of PDMCI. The superior performance of this integrated model underscores the significant potential of eye tracking and gait features for implementing cost-effective screening methods. Future research should further validate the cross-centre applicability of the model and investigate its practical utility in disease intervention and prognosis evaluation scenarios, thereby providing comprehensive technical support for the management of PDMCI patients.
Methods
Study participants
From March 2024 to October 2024, 50 patients diagnosed with PDMCI, 50 patients with PDNC, and 20 healthy controls (HCs) were prospectively recruited. All the participants were from inpatient and outpatient divisions of the Second Department of Neurology at the First Affiliated Hospital of Kunming Medical University. The requirements for PD patients to be included adhered strictly to the diagnostic guidelines established in 2015 by the International Parkinson and Movement Disorder Society (MDS)2. The Type I diagnostic standards for PDMCI, which were released by the MDS in 2012, were used to evaluate patients for PDMCI33. Patients who did not meet the diagnostic standards for PDD or PDMCI were included in the PDNC group34. The Montreal Cognitive Assessment (MoCA) tool was used to evaluate cognitive function in patients with PD35. Patients with MoCA scores > 26 were categorized into the PDNC group, whereas those with scores greater than or equal to 18 but less than 26 were classified into the PDMCI group. The HCs also underwent examinations and were confirmed to have no history of cognitive dysfunction or psychiatric disorders. The study participants were all right-handed.
The following were the exclusion criteria: (1) atypical or secondary symptoms of PD, including progressive supranuclear palsy, multiple-system atrophy, parkinsonism induced by antipsychotic drugs, and other associated disorders; (2) histories of psychiatric disorders, epilepsy, head trauma, brain surgery, or cerebrovascular accidents; (3) ocular conditions such as colour blindness, severe visual impairment, diplopia, cataracts, or prior ocular surgery; (4) moderate to severe occipital tremors or significant limb instability that impaired task performance; (5) factors affecting gait, including fractures and joint injuries; and (6) an inability to adhere to the protocols for head magnetic resonance imaging.
The Ethics Committee of the First Affiliated Hospital of Kunming Medical University granted approval to this investigation (2023-L-54). Each participant provided written informed consent, and the study followed the guidelines set forth in the Belmont Report of the Department of Health and Human Services and in the Declaration of Helsinki of the World Medical Association.
Clinical scale assessment
Demographic and clinical data, including age, sex, educational degree, duration of the illness, and levodopa equivalent daily dose (LEDD), were systematically collected from all the participants. For PD patients, UPDRS-III, Hoehn-Yahr staging, the Freezing of Gait Questionnaire (FOGQ), the Parkinson’s Disease Questionnaire (PDQ-39), and the Activities of Daily Living (ADL) scale were utilized to assess motor symptoms. Mood was evaluated using the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS). Progression of nonmotor symptoms and treatment responses were quantitatively assessed using the Parkinson’s Disease Non-Motor Symptoms Scale (NMSS). Sleep quality and abnormal sleep behaviours were evaluated using the Pittsburgh Sleep Quality Index (PSQI) and the Rapid Eye Movement Sleep Behaviour Disorder Screening Questionnaire (RBDSQ). The Clinical Scale of Sialorrhea in Parkinson’s Disease (SCS-PD) was utilized to assess the severity and associated discomfort of sialorrhea experienced by patients with PD.
As part of this investigation, two skilled neurologists independently assessed the cognitive function of each patient to ensure the accuracy and dependability of the cognitive status assessment. This approach minimized subjective biases and ensured the scientific validity and reliability of the scale assessment results. Additionally, two attending physicians who had received standardized training thoroughly examined each scale assessment result to ensure the accuracy of the measurement data.
Experimental design and data acquisition for a dual-task gait analysis
In this study, a wearable system for the quantitative evaluation of motion and gain based on the MATRIX series (GYENNO SCIENCE) was used to collect gait parameters for three classic paradigms under dual-task conditions: dual-task timed up-and-go, dual-task narrow path, and dual-task circular movements. During the experiment, real-time spatial movement and behavioural data were captured using ten high-precision sensors that were strategically placed on the chest, the waist, the left and right wrists, the thighs, the ankles, and the feet of the subject. Prior to conducting the assessment, standardized height, thigh length (from the hipbone to mid-knee), and calf length (from mid-knee to the ground) measurements were obtained to account for intersubject stature variations. Additionally, to eliminate the potential influence of levodopa on gait performance, all PD patients were instructed to discontinue levodopa intake 12 hours prior to performing data collection, ensuring that they were in an “off” state during the evaluation. To investigate the impacts of cognitive tasks on gait, all paradigms were implemented under dual-task conditions in which participants walked while performing a cognitive task (successive subtraction of 3 from 100). This approach not only assessed how individuals allocated their attention to maintain gait stability and complete cognitive tasks with limited cognitive resources but also evaluated the allocation of attention to these dual objectives. Additionally, it better simulated real-life scenarios and evaluated the degree of gait automation. The requirements for each paradigm were as follows. (1) For the timed up-and-go test, each participant was required to remain seated in a chair, stand up after 5 seconds, walk straight to a line 10 m ahead, return to the chair upon reaching the finish line, turn around, sit down, and remain still. (2) The narrow path movement was similar to the timed up-and-go test, but during straight walking, each participant was required to navigate a narrow path approximately 1.1 times his or her shoulder width. (3) For the circle paradigm, each participant stood in a designated area; he or she first rotated twice continuously to the left, then returned to the starting direction, stood still for 10 s, rotated twice continuously to the right, returned to the starting direction, and remained still.
Experimental design and data acquisition for single-target and multitarget eye tracking studies
The EyeLink Experiment Builder (EB) was used to design the experimental protocol and present the visual stimuli. Data Viewer (DV) was employed for data visualization and analysis purposes. The EyeLink system comprises four essential components, namely, an infrared light source, a high-speed camera, a host computer, and eye-tracking software. The system directs the camera at the participant’s eye, determines the position of the pupil and performs corneal reflection (CR) using image processing techniques that are based on threshold segmentation algorithms; it then tracks ocular movements by monitoring changes in the relative positions of the pupil and the CR. The gaze position is subsequently calculated. The laboratory setup was as follows: an L-shaped configuration was used to ensure a quiet and well-lit environment that was conducive to obtaining accurate measurements. The standard procedures for this experiment included launching the test program, adjusting the camera angle via Global View, fine-tuning the camera focus, setting the pupil and CR thresholds, conducting a four-point check, performing nine-point calibration and verification (the average error was less than 0.5°, and the maximum error was less than 1°), presenting the test stimuli, and initiating data recording.
In the single-target eye-tracking test, a pair of red and green dots initially appeared at random on the screen’s left or right side (Fig. 3a)36. After one second, the green dot changed to a red dot and began to rotate. The direction of dot rotation (either clockwise or counterclockwise) was randomized, and the direction was reset every quarter cycle. Three seconds later, the dots ceased moving, and one of them reverted to green. When the trial was over, the green dot could be either the initial green target or the initial red distractor. After determining if the final green dot matched the first green goal, participants needed to hit the “yes” or “no” button to indicate their response. The angular velocities were adjusted every five trials, and four different angular velocities (4.3 degrees/s, 8.5 degrees/s, 17.2 degrees/s, and 34 degrees/s) were tested over twenty trials. The accuracy attained at different speeds served as the main outcome measure. The multitarget eye-tracking task involved two targets, as shown in Fig. 3b, and significantly increased the complexity of the experiment. At the beginning of every trial, each of the four quadrants on the screen had a pair of points. Among them, one green target point appeared in each of any two sets of pairs, and the remaining six red dots were used as disruptors. The angular velocity for the 30 trials was set to 12.8 degrees per second. The speed of rotation in the single-target eye-tracking task was established based on theoretical considerations and prior research36 and aimed to encompass a spectrum of difficulty from low to high. The rotation speeds were calibrated according to the participants’ abilities to track the target successfully under various test conditions. It was observed that there was a maximum speed threshold at which subjects could effectively track targets and that this threshold was closely associated with the speed and number of the targets, as well as with spatial factors such as proximity to distractors. For the multitarget eye-tracking task, a moderate speed was selected to balance task difficulty with the participants’ capabilities, ensuring that the task remained challenging yet feasible.
Acquisition of neuroimaging data
The radiology department of our hospital used a 3.0-T whole-body scanner (Discovery 750w; GE Healthcare, Fairfield, CT, USA) to obtain all MRI data. A standard head coil was employed for both sending and receiving. Prior to scanning, each participant received detailed instructions regarding the requirements and was asked to lie supine, remain awake, and maintain a state of rest without actively engaging in specific thought processes. To minimize external disturbances, the subjects wore earplugs and were instructed to keep their heads as still as possible to reduce motion artefacts.
A spin-echo planar imaging sequence with 30 gradient directions (b value = 1000 s/mm2) was used to perform diffusion tensor imaging (DTI). The acquisition parameters included an axial interleaved scan with 67 slices; the repetition time (TR) = 8000 ms; the echo time (TE) = 80 ms; the flip angle (FA) = 90°; the field of view (FOV) = 256 × 256 mm²; the matrix size = 128 × 128; the slice thickness = 2 mm; and no slice gap.
A sequence known as echo-planar imaging (EPI) was used to gather RS-fMRI data. The following parameters were used: 240 volumes; the TR = 2000 ms; the TE = 30 ms; the FA = 90°; the FOV = 224 mm; the voxel size = 3.5 × 3.5 × 4 mm³; the number of volumes = 36; the slice thickness = 3 mm; the interslice gap = 1 mm; and the matrix size = 64 × 64.
Processing of the neuroimaging data
DTI: The DSI Studio software was used to handle the DTI data. Initially, the 4D DTI volume (a DICOM file) was converted into an SRC file. Preprocessing was subsequently conducted using the TOPUP and EDDY correction algorithms to address phase distortions, eddy current effects, and motion artefacts. The Q-space diffeomorphism reconstruction (QSDR) technique, specifically an MNI version of generalized Q-sampling imaging (GQI), was then used to rebuild the pictures. This process facilitated the automatic transfer of the images to MNI space and normalization to the ICBM152_adult template. Colour-coded fractional anisotropy maps (FA maps) and diffusion maps were subsequently generated along the x-, y-, and z-axes. Projection and commissural fibres were identified on the colour-coded FA maps, and spherical regions of interest (ROIs) with diameters of 5 mm were manually delineated in a bilateral manner in these fibre regions at the lateral ventricular body level. A total of two ROIs were placed on each subject’s colour-coded FA map and diffusion map. The diffusion values (Dxx, Dyy, and Dzz) for the left projection and the commissural fibres were subsequently extracted and utilized for ALPS calculations.
gFCD: By using the dcm2niix software (https://www.nitrc.org/projects/dcm2nii/), the original DICOM-formatted resting-state fMRI images were first converted to the Neuroimaging Informatics Technology Initiative (NIfTI) format. Subsequently, an image analysis was conducted on the FCD data via the MATLAB R2024a platform (https://ww2.mathworks.cn/products/matlab-online.html). Preprocessing was performed using DPABI 4.5 (https://rfmri.org/DPABI). Data from the first 10 time points were eliminated to remove artefacts that resulted from the calibration of the scanner and adjustments to the scanning setting. Slice timing correction and head motion realignment were applied to the remaining photographs, with the central slice used as the reference. Furthermore, pictures with displacements larger than 2 mm or rotations greater than 2° in any direction (x, y, z) were not included to reduce the influence of head motion on the functional connectivity study. Data normalization was conducted using the DARTEL template in the Montreal Neurological Institute (MNI) space to ensure anatomical precision across different participants. The normalized rs-fMRI data were resampled to a voxel size of 3 × 3 × 3 mm³, linear drift was removed to eliminate low-frequency signals, and bandpass filtering (0.01–0.08 Hz) was applied to filter out physiological high-frequency noise. Based on the AAL atlas, FCD values were computed for 116 brain areas. A Gaussian kernel with a full width at half maximum (FWHM) of 8 mm was then used for spatial smoothing. Finally, the 116 brain areas were subjected to Random Forest (RF) feature significance selection and LASSO regression.
Statistical analysis and feature selection
The SPSS 27.0 software was used to analyse the differences in the general demographic data, clinical scales, gait, eye-tracking parameters, and imaging indicators among the patients with PDMCI and PDNC and HCs. Separate samples t-tests were used to evaluate differences in normally distributed data between two groups. One-way analysis of variance (ANOVA) was employed for comparisons between three or more groups, and post hoc multiple comparisons were conducted using the least-significant difference (LSD) method. Nonnormally distributed data were analysed using the Mann‒Whitney U test for two-group comparisons and via the Kruskal‒Wallis H test for comparisons of three or more groups, followed by Nemenyi post hoc tests. To control for the overall type I error rate, the p-values derived from all tests were adjusted using Bonferroni correction. Medians (interquartile ranges) are used to present nonnormally distributed data, whereas the means ± standard deviations are used to present normally distributed data. Chi-square tests were used to analyse categorical variables. A p-value of less than 0.05 was taken into consideration for all statistical analyses to indicate statistical significance.
RStudio software (version 2024.12.0 Build 467) was used to perform variable selection via LASSO regression. Sparsity was induced in the feature set through L1 regularization, and cross-validation was conducted using the lambda.min (the λ value corresponding to the minimum error) to determine the optimal regularization strength. Thereafter, the variables selected by LASSO regression were incorporated into the RF model. The RF algorithm was utilized to assess feature importance levels, identify the key variables that exerted the most significant influence on the target variables, and ultimately to select the most critical features for model construction. This methodology aimed to yield a representative and predictive feature combination for the subsequent modelling phase.
Development and evaluation of the model
The data processing and analysis flow of this study is based on the Python language and implemented in the Spyder integrated development environment. The itertools.combinations function was utilized to generate all possible feature combinations, ranging from a single feature to all five features, resulting in a total of 31 nonempty combinations derived from the most critical features selected by the aforementioned five modules. This comprehensive combination strategy aims to systematically explore the impact of different feature combinations on model performance, ensuring that no potential effective feature subsets are overlooked. To ensure the objectivity of model evaluation and the effectiveness of hyperparameter optimization, this study adopts a nested cross-validation strategy. The outer cross-validation is used to evaluate the generalization performance of the model, while the inner cross-validation is used for hyperparameter optimization. This structure effectively avoids the problem of hyperparameter leakage. The outer layer cross-validation uses 5-fold stratified cross-validation (stratified kfold), and is repeated 20 times (n_repeats=20), totaling 100 experiments. In each experiment, the dataset was divided into five subsets to ensure that the proportion of each category in each subset was consistent with that of the original dataset. Through multiple repetitions, the stability and reliability of the assessment results have been further enhanced. In the inner layer cross-validation, StratifiedKFold cross-validation is also adopted to optimize the hyperparameters of the SVM model. Its random seed parameter is set to random_state=42. Hyperparameters include the regularization parameter C (0.1,1,10,100), the kernel function parameter γ (1,0.1,0.01,0.001), and the kernel function type (radial basis function kernel, RBF). Through GridSearchCV, the system searches for the optimal combination of hyperparameters in the inner cross-validation to ensure the best performance of the model under the current feature combination and data partitioning. Each outer cross-validation partition does not have a fixed random seed (random_state=None) to ensure the randomness and independence of each partition. This strategy avoids potential biases caused by fixed divisions, making the model evaluation results more universal and representative. In each iteration of outer cross-validation, the test set is completely independent of the training process and does not participate in any model training or hyperparameter optimization. In this way, it is ensured that the test set remains in an untouched state throughout the training and optimization process, thereby effectively avoiding the problem of hyperparameter leakage.
Interpretation of the model
To enhance the interpretability of the model, this study uses SHAP (SHapley Additive exPlanations) analysis to explain the general model. The SHAP method is based on the Shapley value in game theory, providing an intuitive quantitative explanation of the contribution of each feature to the model’s prediction. By calculating the SHAP value of each feature to measure its impact on the model’s prediction results, the complex interactions among features can be revealed. This not only helps to deeply understand the decision-making logic of the model, but also provides valuable guidance for subsequent feature engineering and model optimization.
Data availability
The data supporting the conclusions of this article are included within the article and its additional files. Additional data are available from the corresponding author upon request.
Code availability
The code used in this study can be obtained from the corresponding author upon reasonable request.
References
Deliz, J. R., Tanner, C. M. & Gonzalez-Latapi, P. Epidemiological evidence for an immune component of Parkinson’s Disease[J]. Curr. Neurol. Neurosci. Rep. 24, 163–179 (2022).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease[J]. Mov. Disord. 30, 1591–1601 (2015).
Kholin, V. et al. Cognitive disorders in Parkinson’s disease[J]. Ageing Longevity, 2024, 2709-0205 (2024).
Abraham, D. S. et al. Sex differences in Parkinson’s disease presentation and progression[J]. Parkinsonism Relat. Disord. 69, 48–54 (2019).
Fernández-Lago, H. et al. Cognitive reserve and executive functions in dual task gait performance in Parkinson’s disease[J]. Exp. Brain Res. 242, 2271–2278 (2024).
Li, H. et al. Combined diagnosis for Parkinson’s disease via gait and eye movement disorders[J]. Parkinsonism Relat. Disord. 123, 106979 (2024).
Tang, H. et al. Analysis of gait characteristics and related factors in patients with Parkinson’s disease based on wearable devices[J]. Brain Behav. 14, e3440 (2024).
Tsitsi, P. et al. Fixation duration and pupil size as diagnostic tools in Parkinson’s Disease[J]. J. Parkinsons Dis. 11, 865–875 (2021).
Zhu, Y. et al. X. Multimodal neuroimaging-based prediction of Parkinson’s disease with mild cognitive impairment using machine learning technique[J]. NPJ Parkinsons Dis. 10, 218 (2024). 218: 10(11).
Ding, X.-B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease[J]. Nat. Med. 27, 411–418 (2021).
Taoka, T. et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases[J]. Jpn J. Radio. 35, 172–178 (2017).
Jing-Cai, M. et al. Correlation of glymphatic system abnormalities with Parkinson’s disease progression: a clinical study based on non-invasive fMRI[J]. J. Neurol. 271, 457–471 (2023).
Bae, Y. J. et al. Altered brain glymphatic flow at diffusion-tensor MRI in rapid eye movement sleep behavior disorder[J]. Radiology 307, e221848 (2023).
Gupta R., Kumari S., Senapati A., Ambasta R. K., Kumar P. New era of artificial intelligence and machine learning-based detection, diagnosis, and therapeutics in Parkinson’s disease[J]. Ageing Res. Rev. 90, 102013 (2023).
Almgren, H. et al. Machine learning-based prediction of longitudinal cognitive decline in early Parkinson’s disease using multimodal features[J]. Sci. Rep. 13, 13193 (2023).
Booth, S., Park, K. W., Lee, C. S. & Ko, J. H. Predicting cognitive decline in Parkinson’s disease using FDG-PET–based supervised learning[J]. J. Clin. Invest 132, e157074 (2022).
Gorji A., Jouzdani A. F. Machine learning for predicting cognitive decline within five years in Parkinson’s disease: Comparing cognitive assessment scales with DAT SPECT and clinical biomarkers[J]. PLoS One. 19, e0304355 (2024).
BaiYuan, Y. et al. Machine learning model base on metabolomics and proteomics to predict cognitive impairment in Parkinson’s disease[J]. npj Parkinson’s. Dis. 10, 187 (2024).
Chen, B. et al. Detection of mild cognitive impairment in Parkinson’s disease using gradient boosting decision tree models based on multilevel DTI indices[J]. J. Transl. Med. 21, 310 (2023).
Mohammadi, R. et al. Machine learning for early detection of cognitive decline in Parkinson’s disease using multimodal biomarker and clinical data[J]. Biomedicines. 12, 2758 (2024).
Johansson, M. E., Toni, I., Kessels, R. P. C., Bloem, B. R. & Helmich, R. C. Clinical severity in Parkinson’s disease is determined by decline in cortical compensation[J]. Brain 147, 871–886 (2024).
Cao, S.-S., Yuan, X.-Z., Wang, S.-H., Taximaimaiti, R. & Wang, X.-P. Transverse strips instead of wearable laser lights alleviate the sequence effect toward a destination in Parkinson’s disease patients with freezing of Gait[J]. Front. Neurol. 11, 838 (2020).
Kinugawa, K., Mano, T. & Sugie, K. Changes in brain functional connectivity between on and off states and their relationship with cognitive impairment in Parkinson’s disease[J]. Sci. Rep. 14, 27333 (2024).
Yeager, B. E., Twedt, H. P., Bruss, J., Schultz, J. & Narayanan, N. S. Cortical and subcortical functional connectivity and cognitive impairment in Parkinson’s disease[J]. Neuroimage Clin. 42, 103610 (2024).
Bologna, M. et al. Facial bradykinesia[J]. J. Neurol. Neurosurg. Psychiatry 84, 681–685 (2013).
Fitzpatrick, E., Hohl, N., Silburn, P., O’Gorman, C. & Broadley, S. A. Case-control study of blink rate in Parkinson’s disease under different conditions[J]. J. Neurol. 259, 739–744 (2012).
Francis, M. et al. Rapid voluntary blinking as a clinical marker of Parkinson’s disease[J]. J. Parkinsons Dis. 14, 993–997 (2024).
Pang, H. et al. Glymphatic function from diffusion-tensor MRI to predict conversion from mild cognitive impairment to dementia in Parkinson’s disease[J]. J. Neurol. 271, 5598–5609 (2024).
Wood, K. H. et al. Diffusion tensor imaging-along the perivascular-space index is associated with disease progression in Parkinson’s disease[J]. Mov. Disord. 39, 1504–1513 (2024).
Liu, X. et al. Cross-vendor test-retest validation of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating glymphatic system function[J]. Aging Dis. 15, 1885–1898 (2023).
Jingyi, L. et al. A detection model of cognitive impairment via the integrated gait and eye movement analysis from a large Chinese community cohort[J]. Alzheimers Dement 20, 1089–1101 (2023).
Aymeric, L., Aymeric, B. & Suzanne, L. A machine learning approach for gene prioritization in Parkinson’s disease[J]. Brain 147, 743–745 (2024).
Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines[J]. Mov. Disord. 27, 349–356 (2012).
Emre, M. et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease[J]. Mov. Disord. 22, 1689–1707 (2007).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment[J]. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Norton, D. J. et al. Visuospatial attention to single and multiple objects is independently impaired in Parkinson’s disease[J]. PLoS One 11, e0150013 (2016).
Acknowledgements
This study was funded by the National Natural Science Foundation of China [82560237]; Yunnan Province Clinical Research Center for Neurological Disease [202102AA310061]; The Innovative Team of Yunnan Province [202305AS350019]; Yunnan health training project of high level talents [D-2024035]; Applied Basic Research Foundation of Yunnan Province [202301AS070045].
Author information
Authors and Affiliations
Contributions
Conceptualization: L.C.Y., C.Y.L., and Y.X.L. Supervision: Y.X.L. Data curation: C.J.Y. and L.C.X. Writing—original draft: L.C.Y. and C.Y.L., Review and editing: Z.Y.F., Z.Y.Y., W.F., and Y.X.L. Investigation: L.C.Y., C.Y.L., C.J.Y. and L.C.X. All co-authors read and approved the document.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, C., Chen, Y., Zhu, Y. et al. Construction of a mild cognitive impairment prediction model for Parkinson’s disease patients on the basis of multimodal data. npj Parkinsons Dis. 11, 318 (2025). https://doi.org/10.1038/s41531-025-01172-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01172-z